Development of an Ex Vivo Assay for Identification of Infectious Hepatitis E Virus in Different Kinds of Food Samples
Abstract
:1. Introduction
2. Materials and Methods
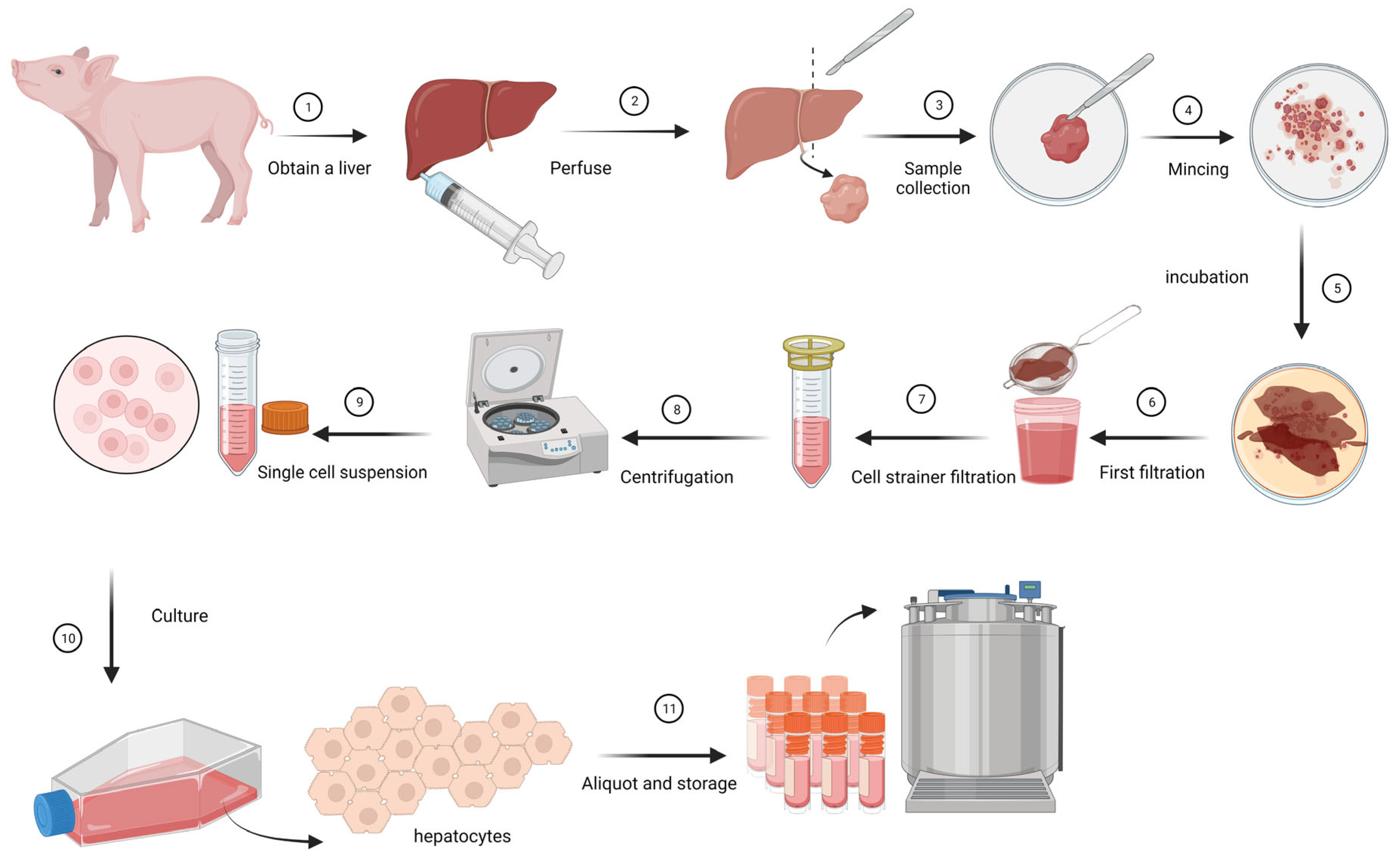
2.1. Isolation and Growing of Primary Hepatocytes
2.2. Immune Peroxidase Monolayer Assay (IPMA)
2.3. Inoculation of Primary Hepatocytes
2.4. RNA Extraction/Isolation and Real-Time RT-PCR
2.5. Sensitivity of the System Compared to a Pig Model
3. Results
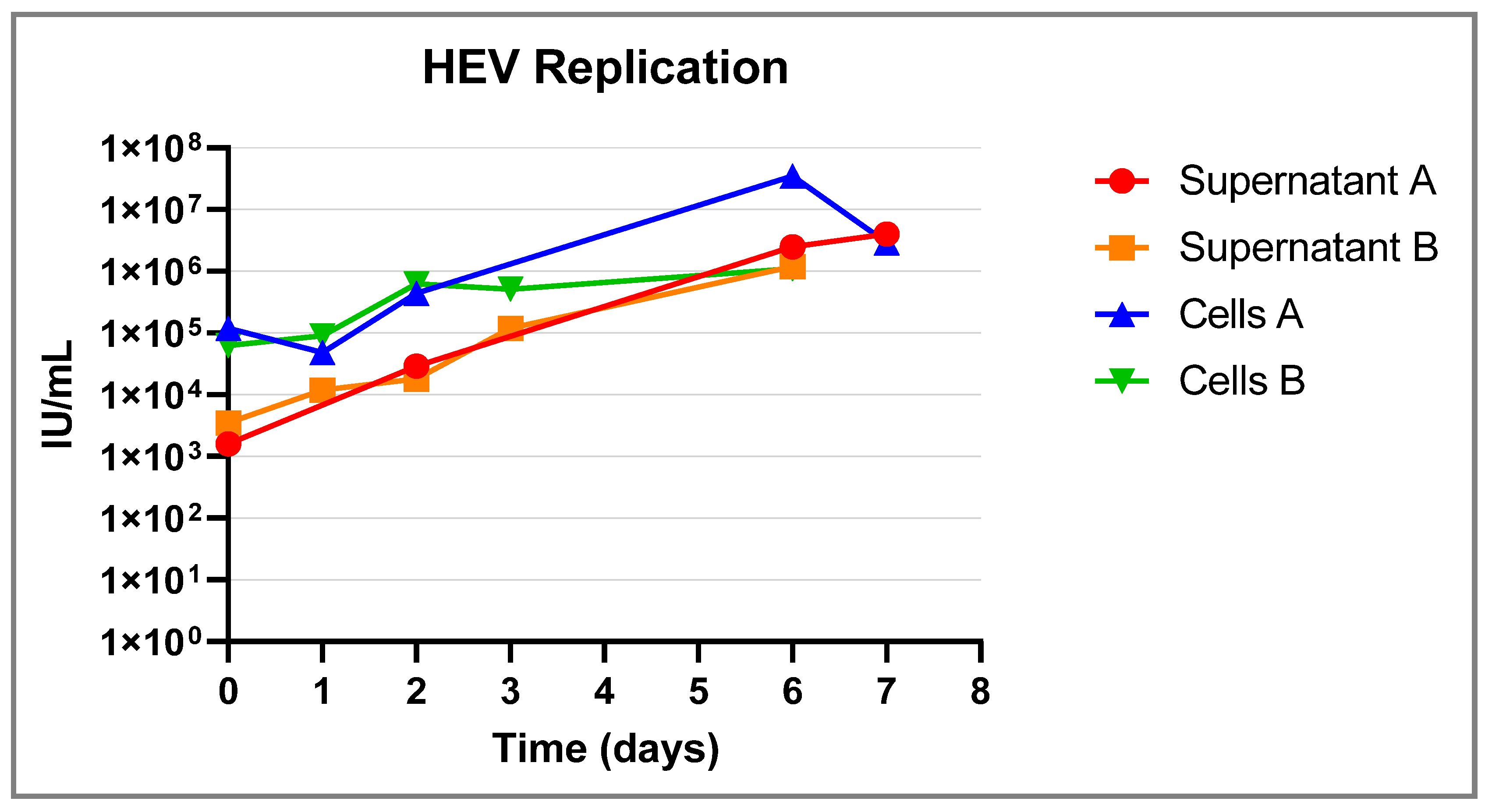
3.1. Isolation and Culturing of the Primary Hepatocytes
3.2. Sensitivity of the System Compared to the Animal Pig Model
| Concentration of the Inoculum (Genomic Copies/mL) | Concentration of the Inoculum (Log Genomic Copies/mL) | Log Genome Copies/mL at D0 | Log Genome Copies/mL at D6 | Primary Hepatocytes Model (Increase Log Copies/mL at D6 after Inoculation) | Animal Model (Total Animals/Tested Positive) |
|---|---|---|---|---|---|
| 107 | 7.00 | 1.29 (SD = 0.00) | 5.14 (SD = 0.12) | 3.47 (SD = 0.66) | 6/6 |
| 4 × 105 | 5.60 | 1.50 (SD = 0.30) | 4.32 (SD = 0.01) | 3.52 (SD = 0.38) | Nt |
| 8 × 104 | 4.90 | 1.30 (SD = 0.02) | 4.32 (SD = 0.01) | 3.02 (SD = 0.03) | Nt |
| 104 | 4.00 | 1.68 (SD = 0.46) | 3.33 (SD = 0.06) | 1.65 (SD = 0.52 | 6/6 |
| 3.33 × 103 | 3.52 | 1.29 (SD = 0.00) | 2.73 (SD = 0.73) | 1.45 (SD = 0.34) | Nt |
| 2 × 103 | 3.30 | 1.29 (SD = 0.00) | 2.16 (SD = 0.15) | 0.87 (SD = 0.15) | Nt |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Hepatitis E; WHO: Geneva, Switzerland, 2021.
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.-J.; Norder, H.; Okamoto, H.; van der Poel, W.H.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E virus infection. Nat. Rev. Dis. Primers 2017, 3, 17086. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-J. Expanding Host Range and Cross-Species Infection of Hepatitis E Virus. PLoS Pathog. 2016, 12, e1005695. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Tan, B.-H.; Teo, E.C.-Y.; Lim, S.-G.; Dan, Y.-Y.; Wee, A.; Aw, P.P.K.; Zhu, Y.; Hibberd, M.L.; Tan, C.-K.; et al. Chronic Infection with Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357.e3. [Google Scholar] [CrossRef]
- Lhomme, S.; Marion, O.; Abravanel, F.; Izopet, J.; Kamar, N. Clinical Manifestations, Pathogenesis and Treatment of Hepatitis E Virus Infections. J. Clin. Med. 2020, 9, 331. [Google Scholar] [CrossRef]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Péron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Faculty Opinions recommendation of Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; et al. Scientific opinion on the public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. 2017, 15, e04886. [Google Scholar]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig Liver Sausage as a Source of Hepatitis E Virus Transmission to Humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef]
- Berto, A.; Grierson, S.; der Honing, R.H.-V.; Martelli, F.; Johne, R.; Reetz, J.; Ulrich, R.G.; Pavio, N.; Van der Poel, W.H.; Banks, M. Hepatitis E Virus in Pork Liver Sausage, France. Emerg. Infect. Dis. 2012, 19, 264–266. [Google Scholar] [CrossRef]
- Feagins, A.R.; Opriessnig, T.; Guenette, D.K.; Halbur, P.G.; Meng, X.-J. Detection and characterization of infectious Hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J. Gen. Virol. 2007, 88, 912–917. [Google Scholar] [CrossRef]
- Cook, N.; Van der Poel, W.H. Survival and Elimination of Hepatitis E Virus: A Review. Food Environ. Virol. 2015, 7, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Van der Poel, W.H. Food and environmental routes of Hepatitis E virus transmission. Curr. Opin. Virol. 2014, 4, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, S.A.; Lodder, W.J.; Lodder-Verschoor, F.; van den Berg, H.H.; Vennema, H.; Duizer, E.; Koopmans, M.; Husman, A.M.d.R. Sources of Hepatitis E Virus Genotype 3 in The Netherlands. Emerg. Infect. Dis. 2009, 15, 381–387. [Google Scholar] [CrossRef]
- Meester, M.; Bouwknegt, M.; der Honing, R.H.-V.; Vernooij, H.; Houben, M.; van Oort, S.; van der Poel, W.H.M.; Stegeman, A.; Tobias, T. Repeated cross-sectional sampling of pigs at slaughter indicates varying age of hepatitis E virus infection within and between pig farms. Veter. Res. 2022, 53, 50. [Google Scholar] [CrossRef] [PubMed]
- Berto, A.; Van der Poel, W.; der Honing, R.H.-V.; Martelli, F.; La Ragione, R.; Inglese, N.; Collins, J.; Grierson, S.; Johne, R.; Reetz, J.; et al. Replication of hepatitis E virus in three-dimensional cell culture. J. Virol. Methods 2013, 187, 327–332. [Google Scholar] [CrossRef]
- Meister, T.L.; Bruening, J.; Todt, D.; Steinmann, E. Cell culture systems for the study of hepatitis E virus. Antivir. Res. 2019, 163, 34–49. [Google Scholar] [CrossRef]
- Johne, R.; Trojnar, E.; Filter, M.; Hofmann, J. Thermal Stability of Hepatitis E Virus as Estimated by a Cell Culture Method. Appl. Environ. Microbiol. 2016, 82, 4225–4231. [Google Scholar] [CrossRef]
- Schemmerer, M.; Apelt, S.; Trojnar, E.; Ulrich, R.G.; Wenzel, J.J.; Johne, R. Enhanced Replication of Hepatitis E Virus Strain 47832c in an A549-Derived Subclonal Cell Line. Viruses 2016, 8, 267. [Google Scholar] [CrossRef]
- Scholz, J.; Bächlein, C.; Gadicherla, A.K.; Falkenhagen, A.; Tausch, S.H.; Johne, R. Establishment of a Plasmid-Based Reverse Genetics System for the Cell Culture-Adapted Hepatitis E Virus Genotype 3c Strain 47832c. Pathogens 2020, 9, 157. [Google Scholar] [CrossRef]
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol. Methods 2006, 131, 65–71. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Lodder-Verschoor, F.; Van Der Poel, W.H.M.; Rutjes, S.A.; Husman, A.M.D.R. Hepatitis E virus RNA in commercially available porcine livers in The Netherlands. J. Food Prot. 2007, 70, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Hakze-Van Der Honing, R.W.; Van Coillie, E.; Antonis, A.F.; van der Poel, W.H. First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS ONE 2011, 6, e22673. [Google Scholar] [CrossRef] [PubMed]
- Boxman, I.L.; Verhoef, L.; Dop, P.Y.; Vennema, H.; Dirks, R.A.; Opsteegh, M. High prevalence of acute hepatitis E virus infection in pigs in Dutch slaughterhouses. Int. J. Food Microbiol. 2022, 379, 109830. [Google Scholar] [CrossRef]
- Schielke, A.; Filter, M.; Appel, B.; Johne, R. Thermal stability of hepatitis E virus assessed by a molecular biological approach. Virol. J. 2011, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Capelli, N.; Dubois, M.; Pucelle, M.; Da Silva, I.; Lhomme, S.; Abravanel, F.; Chapuy-Regaud, S.; Izopet, J. Optimized Hepatitis E Virus (HEV) Culture and Its Application to Measurements of HEV Infectivity. Viruses 2020, 12, 139. [Google Scholar] [CrossRef]
- Chew, N.; Situ, J.; Wu, S.; Yao, W.; Sridhar, S. Independent Evaluation of Cell Culture Systems for Hepatitis E Virus. Viruses 2022, 14, 1254. [Google Scholar] [CrossRef]
- Chivu, M.; Dima, S.O.; Stancu, C.I.; Dobrea, C.; Uscatescu, V.; Necula, L.G.; Bleotu, C.; Tanase, C.; Albulescu, R.; Ardeleanu, C.; et al. In vitro hepatic differentiation of human bone marrow mesenchymal stem cells under differential exposure to liver-specific factors. Transl. Res. 2009, 154, 122–132. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Teunis, P.F.M.; Frankena, K.; de Jong, M.C.M.; Husman, A.M.d.R. Estimation of the Likelihood of Fecal-Oral HEV Transmission Among Pigs. Risk Anal. 2010, 31, 940–950. [Google Scholar] [CrossRef]
- Andraud, M.; Dumarest, M.; Cariolet, R.; Aylaj, B.; Barnaud, E.; Eono, F.; Pavio, N.; Rose, N. Direct contact and environmental contaminations are responsible for HEV transmission in pigs. Veter. Res. 2013, 44, 102. [Google Scholar] [CrossRef]

| Origin (Sample Type and HEV Genotype) | Log Genome Copies/mL of the Tested Sample | Country of Origin | Reference | Log Genome Copies/mL at D0 | Log Genome Copies/mL at D6 | Log Increase in Genome Copies/mL |
|---|---|---|---|---|---|---|
| Positive control (HEV PCR-positive hepatocytes HEV-3f) | 6.42 | The Netherlands | WBVR | 1.99 (SD = 0.68) | 5.44 (SD = 0.14) | 3.45 (SD = 0.54) |
| Liver HEV-3f | 5.10 | The Netherlands | Bouwknegt et al. [22] | 2.96 (SD = 0.68) | 5.1 (SD = 0.23) | 2.06 (SD = 0.91) |
| Liver HEV-4 | 3.66 | Belgium | Hakze et al. [23] | 1.29 (SD = 0.00) | 2.51 (SD = 0.07) | 1.22 (SD = 0.07) |
| Liver HEV-3f in different dilutions | 7.37 | Denmark | WBVR | See Table 2 | See Table 2 | See Table 2 |
| Sausage | 3.49 | France | Berto et al. [10] | 1.29 (SD = 0.00) | 2.5 (SD = 0.00) | 1.21 (SD = 0.00) |
| Faeces HEV-3c | 6.49 | The Netherlands | WBVR, 2019 | 3.58 (SD = 0.09) | 4.67 (0.27) | 1.1 (SD = 0.17) |
| Liver 2019A HEV-3c | 7.16 | The Netherlands | Boxman et al. [24] | 3.14 (SD = 0.25) | 4.04 (SD = 0.06) | 0.63 (SD = 0.19) |
| Liver 2019B HEV-3c | 6.39 | The Netherlands | Boxman et al. [24] | 3.04 (SD = 0.14) | 3.52 (SD = 0.11) | 0.48 (SD = 0.03) |
| Liver 2022A HEV-3c | 4.39 | The Netherlands | WFSR 2022 | 1.36 (SD = 0.10) | 2.45 (SD = 0.07) | 1.09 (SD = 0.03) |
| Liver 2022B HEV-3c | 6.58 | The Netherlands | WFSR 2022 | 2.64 (SD = 0.47) | 3.16 (SD = 0.02) | 0.52 (SD = 0.49) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakze-van der Honing, R.W.; van Oort, S.; Dirks, R.A.M.; van der Poel, W.H.M. Development of an Ex Vivo Assay for Identification of Infectious Hepatitis E Virus in Different Kinds of Food Samples. Pathogens 2023, 12, 1231. https://doi.org/10.3390/pathogens12101231
Hakze-van der Honing RW, van Oort S, Dirks RAM, van der Poel WHM. Development of an Ex Vivo Assay for Identification of Infectious Hepatitis E Virus in Different Kinds of Food Samples. Pathogens. 2023; 12(10):1231. https://doi.org/10.3390/pathogens12101231
Chicago/Turabian StyleHakze-van der Honing, Renate W., Sophie van Oort, René A. M. Dirks, and Wim H. M. van der Poel. 2023. "Development of an Ex Vivo Assay for Identification of Infectious Hepatitis E Virus in Different Kinds of Food Samples" Pathogens 12, no. 10: 1231. https://doi.org/10.3390/pathogens12101231





