Toward Mechanistic Design of Surrogate Buffers for Dissolution Testing of pH-Dependent Drug Delivery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing of EC Hard Shell Capsules
2.3. Dissolution Test
2.4. Buffer Preparation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Dissolution of Mesalamine Tablets Coated with a Mixture of Methacrylic Acid/Methyl Methacrylate Copolymer (1:2) and Methacrylic Acid/Methyl Methacrylate Copolymer (1:1)
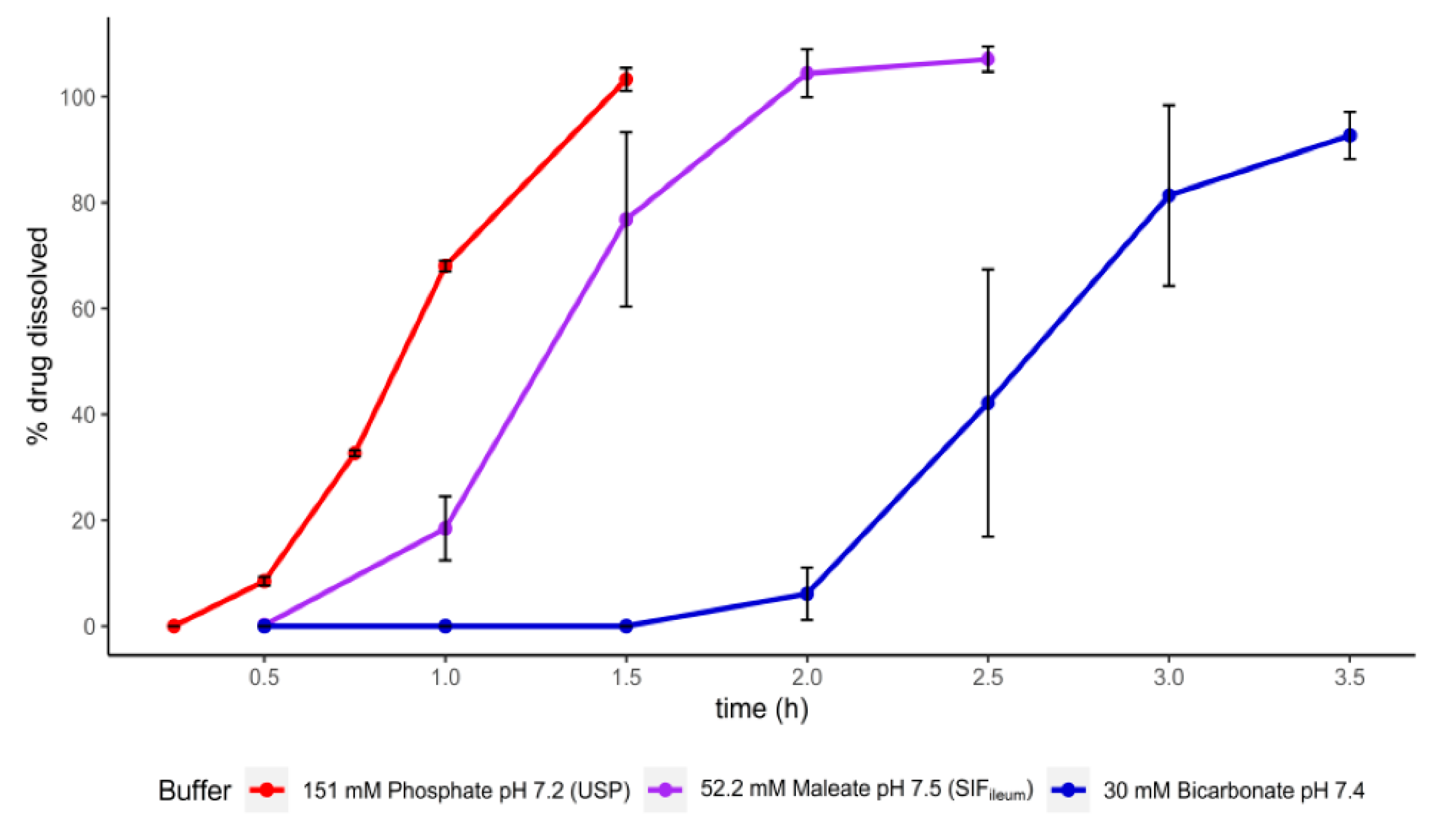
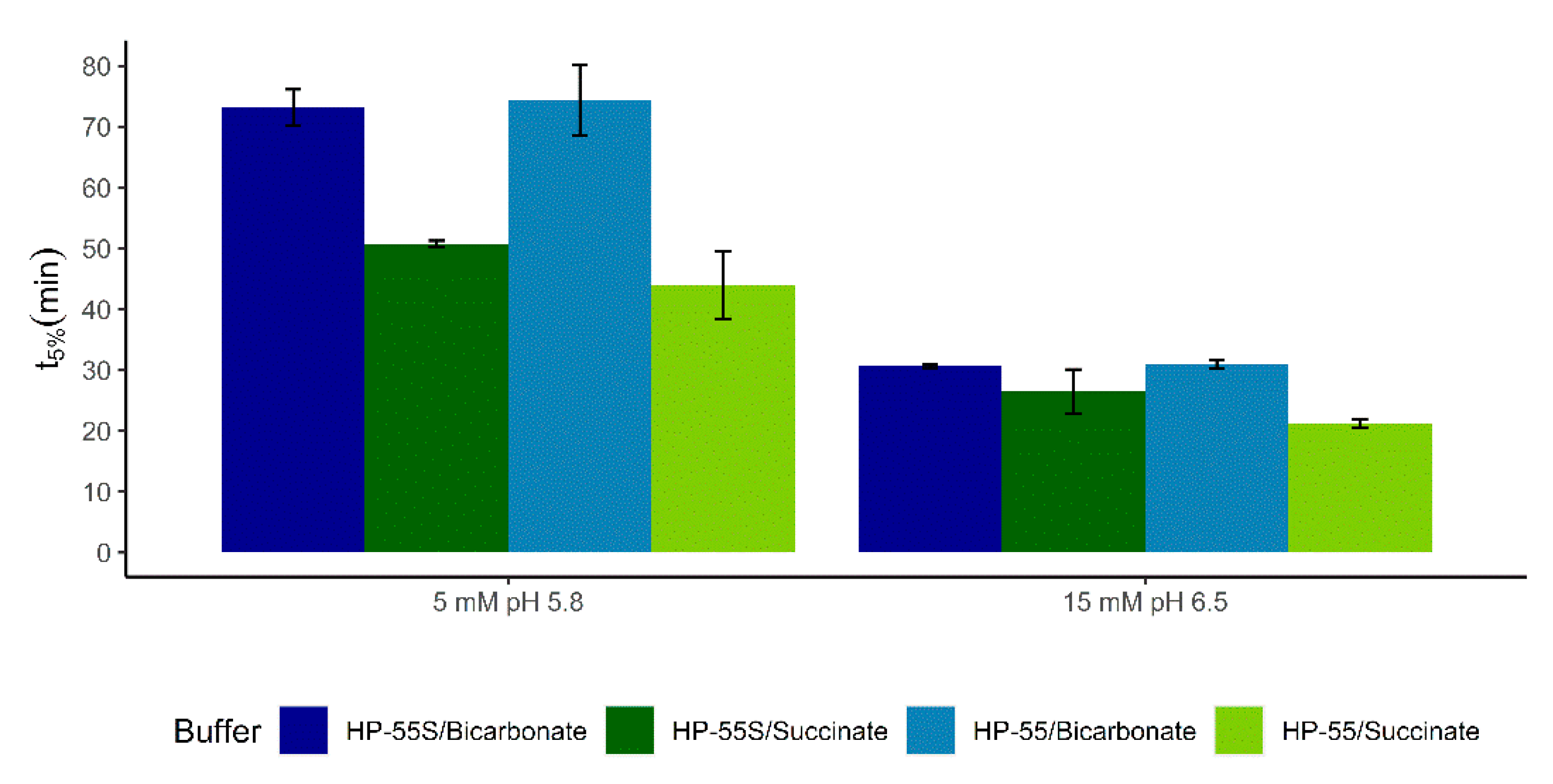
3.2. Dissolution of Paracetamol Capsules Coated with Eudragit S, HP-55, and HP-55S
3.3. Dissolution of Paracetamol Capsules Coated with HPMCAS-LF and Caffeine Capsules Coated with HP-50
3.4. Dissolution of Diclofenac Sodium Tablets Coated with Methacrylic Acid/Ethyl Acrylate Copolymer (1:1)
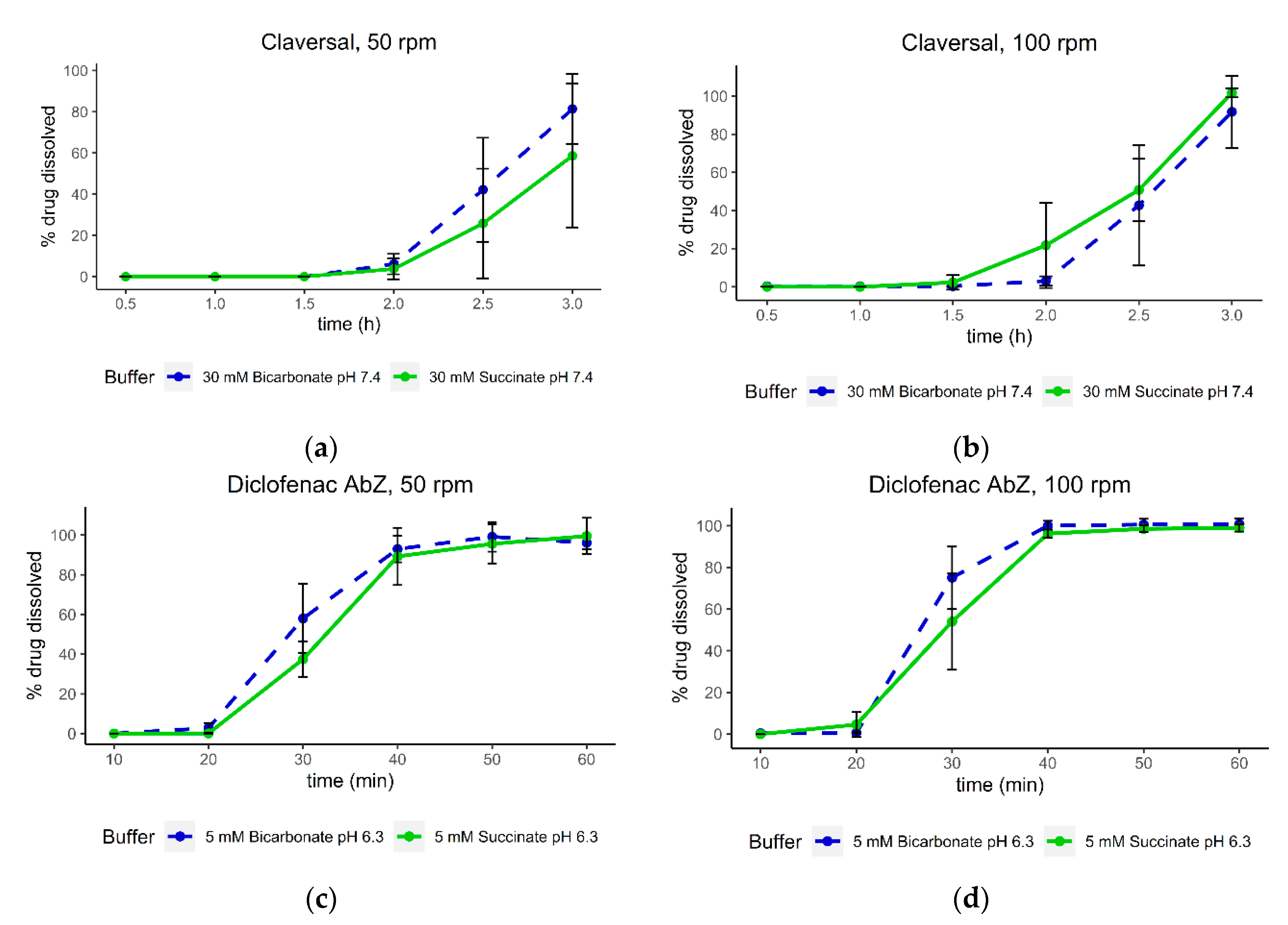
3.5. Dissolution of Claversal Tablets and Diclofenac AbZ Tablets with Increased Agitation Speed
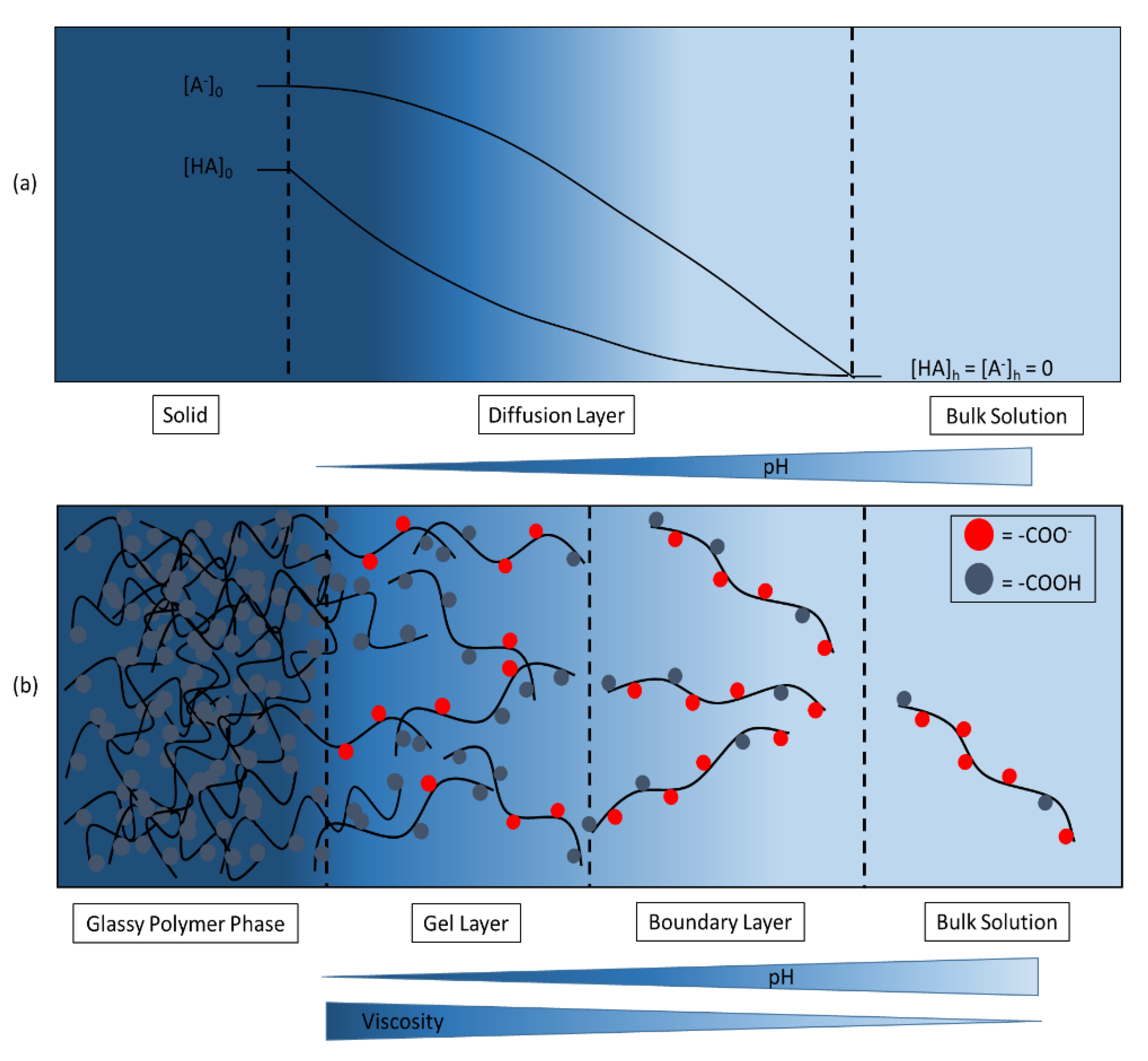
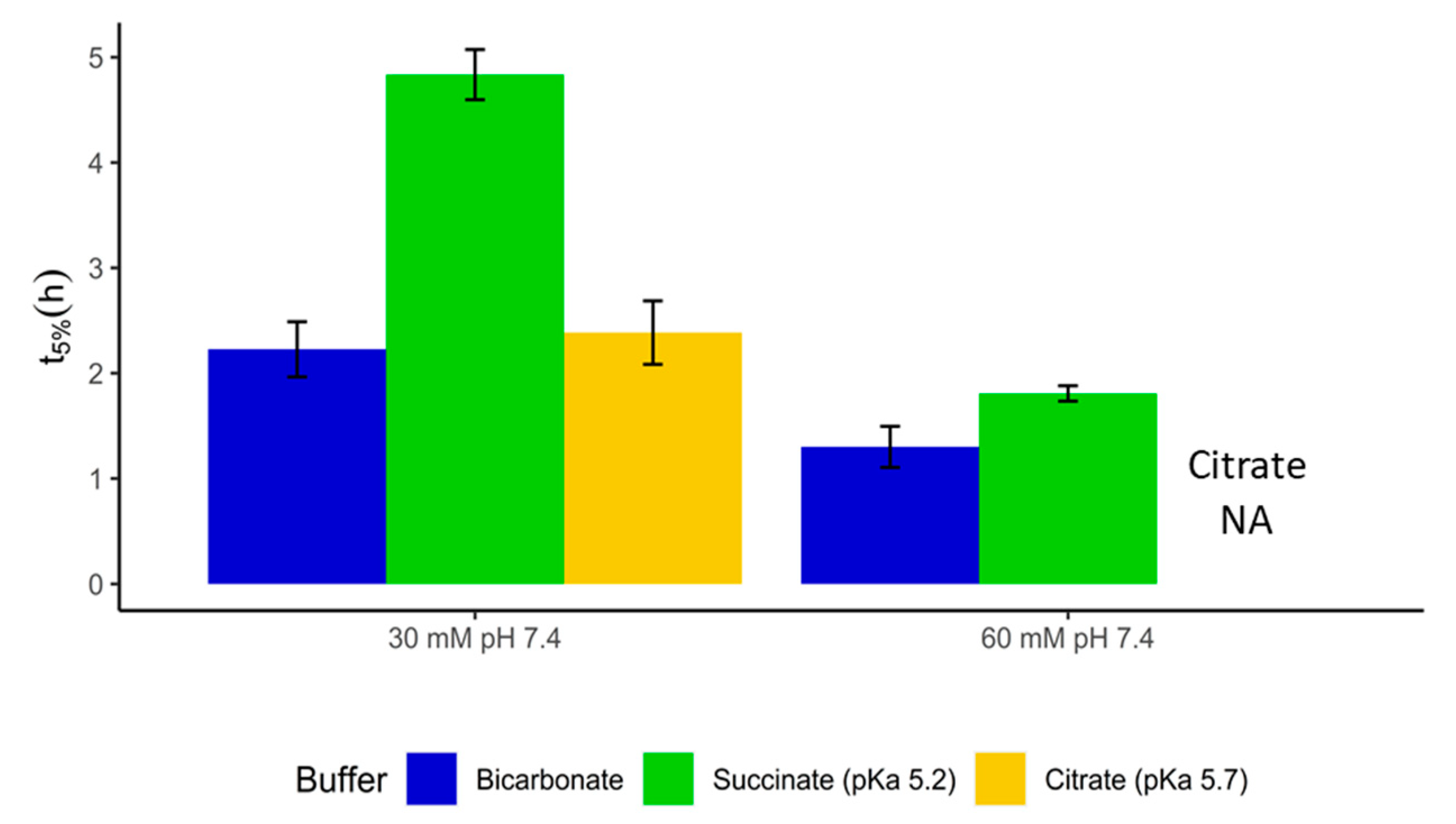
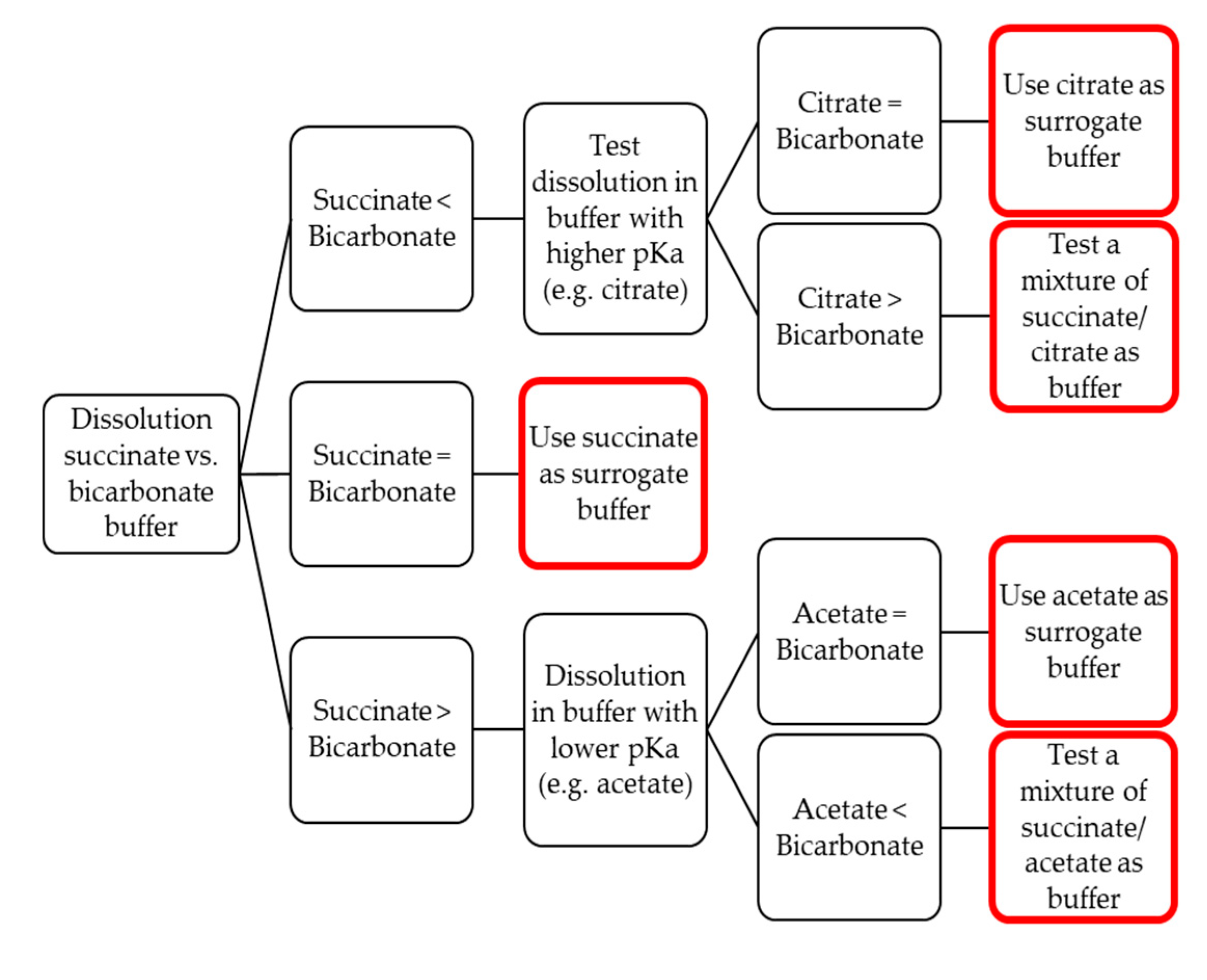
3.6. Establishing Surrogate Buffers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Gousous, J.; Tsume, Y.; Fu, M.; Salem, I.I.; Langguth, P. Unpredictable Performance of pH-Dependent Coatings Accentuates the Need for Improved Predictive in Vitro Test Systems. Mol. Pharm. 2017, 14, 4209–4219. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Amidon, G.L.; Reppas, C.; Shah, V.P. Dissolution Testing as a Prognostic Tool for Oral Drug Absorption: Immediate Release Dosage Forms. Pharm. Res. 1998, 15, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudie, D.M.; Amidon, G.L.; Amidon, G.E. Physiological Parameters for Oral Delivery andin VitroTesting. Mol. Pharm. 2010, 7, 1388–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadda, H.M.; Basit, A.W. Dissolution of pH responsive formulations in media resembling intestinal fluids: Bicarbonate versus phosphate buffers. J. Drug Deliv. Sci. Technol. 2005, 15, 273–279. [Google Scholar] [CrossRef]
- Amaral Silva, D.A.; Davies, N.M.; Doschak, M.R.; Al-Gousous, J.; Bou-Chacra, N.; Löbenberg, R. Mechanistic understanding of underperforming enteric coated products: Opportunities to add clinical relevance to the dissolution test. J. Control. Release 2020, 325, 323–334. [Google Scholar] [CrossRef] [PubMed]
- USP. The United States Pharmacopoeia and National Formulary, 42th ed.; United States Pharmacopoeia Convention: Rockville, MA, USA, 2019. [Google Scholar]
- Fadda, H.M.; Merchant, H.A.; Arafat, B.T.; Basit, A.W. Physiological bicarbonate buffers: Stabilisation and use as dissolution media for modified release systems. Int. J. Pharm. 2009, 382, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Al-Gousous, J.; Sun, K.X.; McNamara, D.P.; Hens, B.; Salehi, N.; Langguth, P.; Bermejo, M.; Amidon, G.E.; Amidon, G.L. Mass Transport Analysis of the Enhanced Buffer Capacity of the Bicarbonate–CO2Buffer in a Phase-Heterogenous System: Physiological and Pharmaceutical Significance. Mol. Pharm. 2018, 15, 5291–5301. [Google Scholar] [CrossRef]
- Al-Gousous, J.; Salehi, N.; Amidon, G.E.; Ziff, R.M.; Langguth, P.; Amidon, G.L. Mass Transport Analysis of Bicarbonate Buffer: Effect of the CO2–H2CO3 Hydration–Dehydration Kinetics in the Fluid Boundary Layer and the Apparent Effective pKa Controlling Dissolution of Acids and Bases. Mol. Pharm. 2019, 16, 2626–2635. [Google Scholar] [CrossRef]
- Krieg, B.J.; Taghavi, S.M.; Amidon, G.L.; Amidon, G.E. In Viv o Predictive Dissolution: Transport Analysis of the CO 2, Bicarbonate In Vivo Buffer System. J. Pharm. Sci. 2014, 103, 3473–3490. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, M.; García, M.A.; Al-Gousous, J.; Ruiz-Picazo, A.; Thieringer, F.; Nguyen, M.A.; Månsson, W.; Galle, P.R.; Langguth, P. In vitro prediction of in vivo absorption of ibuprofen from suspensions through rational choice of dissolution conditions. Eur. J. Pharm. Biopharm. 2020, 149, 229–237. [Google Scholar] [CrossRef]
- Al-Gousous, J.; Amidon, G.L.; Langguth, P. Toward Biopredictive Dissolution for Enteric Coated Dosage Forms. Mol. Pharm. 2016, 13, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K. Theoretical comparison of hydrodynamic diffusion layer models used for dissolution simulation in drug discovery and development. Int. J. Pharm. 2008, 363, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Catteau, D.; Barthélémy, C.; Deveaux, M.; Robert, H.; Trublin, F.; Marchandise, X.; Van Drunen, H. Contribution of scintigraphy to verify the reliability of different preparation processes for enteric coated capsules. Eur. J. Drug Metab. Pharmacokinet. 1994, 19, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Al-Gousous, J.; Blechar, J.A.; Langguth, P. Enteric Hard Capsules for Targeting the Small Intestine: Positive Correlation between In Vitro Disintegration and Dissolution Times. Pharmaceutics 2020, 12, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Gousous, J.; Ruan, H.; Blechar, J.; Sun, K.; Salehi, N.; Langguth, P.; Job, N.; Lipka, E.; Loebenberg, R.; Bermejo, M.; et al. Mechanistic analysis and experimental verification of bicarbonate-controlled enteric coat dissolution: Potential in vivo implications. Eur. J. Pharm. Biopharm. 2019, 139, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Andreas, C.J.; Chen, Y.-C.; Markopoulos, C.; Reppas, C.; Dressman, J.B. In vitro biorelevant models for evaluating modified release mesalamine products to forecast the effect of formulation and meal intake on drug release. Eur. J. Pharm. Biopharm. 2015, 97, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Banwell, J.G.; Pierce, N.F.; Mitra, R.C.; Brigham, K.L.; Caranasos, G.J.; Keimowitz, R.I.; Fedson, D.S.; Thomas, J.; Gorbach, S.L.; Sack, R.B.; et al. Intestinal fluid and electrolyte transport in human cholera. J. Clin. Investig. 1970, 49, 183–195. [Google Scholar] [CrossRef]
- Mooney, K.; Mintun, M.; Himmelstein, K.; Stella, V. Dissolution Kinetics of Carboxylic Acids II: Effect of Buffers. J. Pharm. Sci. 1981, 70, 22–32. [Google Scholar] [CrossRef]
- Sheng, J.J.; McNamara, D.P.; Amidon, G.L. Toward anIn VivoDissolution Methodology: A Comparison of Phosphate and Bicarbonate Buffers. Mol. Pharm. 2009, 6, 29–39. [Google Scholar] [CrossRef] [Green Version]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 444266, Maleic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Maleic-acid (accessed on 1 September 2020).
- Sinko, P.J. Ionic Equilibria. In Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences, 6th ed.; International ed.; 50th anniversary ed.; Sinko, P.J., Ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; p. 161. ISBN 978 1 6091 3402 0. [Google Scholar]
- Amaral Silva, D.A.; Al-Gousous, J.; Davies, N.M.; Bou Chacra, N.B.; Webster, G.K.; Lipka, E.; Amidon, G.; Löbenberg, R. Simulated, biorelevant, clinically relevant or physiologically relevant dissolution media: The hidden role of bicarbonate buffer. Eur. J. Pharm. Biopharm. 2019, 142, 8–19. [Google Scholar] [CrossRef]
- Shibata, H.; Yoshida, H.; Izutsu, K.-I.; Goda, Y. Use of bicarbonate buffer systems for dissolution characterization of enteric-coated proton pump inhibitor tablets. J. Pharm. Pharmacol. 2016, 68, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.A.; Fogler, H.S. Facilitated diffusion in the dissolution of carboxylic polymers. AIChE J. 2005, 51, 415–425. [Google Scholar] [CrossRef]
- Florence, A.T.; Attwood, D. Polymers and Macromolecules. In Physicochemical Principles of Pharmacy: In Manufacture, Formulation and Clinical Use, 6th ed.; Florence, A.T., Attwood, D., Eds.; Pharmaceutical Press: London, UK, 2016; pp. 301–302. ISBN 9780857111746. [Google Scholar]
- McGee, L.C.; Hastings, A.B. The carbon dioxide tension and acid-base balance of jejunal secretions in man. J. Biol. Chem. 1942, 142, 893–904. [Google Scholar]
- Salehi, N.; Al-Gousous, J.; Mudie, D.M.; Amidon, G.L.; Ziff, R.M.; Amidon, G.E. Hierarchical Mass Transfer Analysis of Drug Particle Dissolution, Highlighting the Hydrodynamics, pH, Particle Size and Buffer Effects for Dissolution of Ionizable and non-Ionizable Drugs in a Compendial Dissolution Vessel. Mol. Pharm. 2020, 17, 3870–3884. [Google Scholar] [CrossRef]



| Batch | Dose | Coating Level |
|---|---|---|
| Eudragit S | 220.7 ± 5.3 mg | 5.50 mg/cm2 |
| HPMCAS-LF | 205.7 ± 2.8 mg | 9.16 mg/cm2 |
| HP-55 | 214.7 ± 3.0 mg | 9.04 mg/cm2 |
| HP-55S | 214.7 ± 3.0 mg | 6.92 mg/cm2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blechar, J.A.; Al-Gousous, J.; Wilhelmy, C.; Postina, A.M.; Getto, M.; Langguth, P. Toward Mechanistic Design of Surrogate Buffers for Dissolution Testing of pH-Dependent Drug Delivery Systems. Pharmaceutics 2020, 12, 1197. https://doi.org/10.3390/pharmaceutics12121197
Blechar JA, Al-Gousous J, Wilhelmy C, Postina AM, Getto M, Langguth P. Toward Mechanistic Design of Surrogate Buffers for Dissolution Testing of pH-Dependent Drug Delivery Systems. Pharmaceutics. 2020; 12(12):1197. https://doi.org/10.3390/pharmaceutics12121197
Chicago/Turabian StyleBlechar, Johannes Andreas, Jozef Al-Gousous, Christoph Wilhelmy, Annika Marielina Postina, Marcus Getto, and Peter Langguth. 2020. "Toward Mechanistic Design of Surrogate Buffers for Dissolution Testing of pH-Dependent Drug Delivery Systems" Pharmaceutics 12, no. 12: 1197. https://doi.org/10.3390/pharmaceutics12121197
APA StyleBlechar, J. A., Al-Gousous, J., Wilhelmy, C., Postina, A. M., Getto, M., & Langguth, P. (2020). Toward Mechanistic Design of Surrogate Buffers for Dissolution Testing of pH-Dependent Drug Delivery Systems. Pharmaceutics, 12(12), 1197. https://doi.org/10.3390/pharmaceutics12121197






