Preclinical Assessment of Nebulized Surfactant Delivered through Neonatal High Flow Nasal Cannula Respiratory Support
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surfactant Preparation and Nebulizer
2.2. High Flow Nasal Cannula (HFNC) Devices
2.3. Pharyngeal Pressure Measurements
2.4. Benchmark Surfactant Aerosol Deposition Studies
2.5. In Vivo Effect of Nebulized Surfactant during HFNC with the OptiflowTM System in Surfactant-Depleted Rabbits
2.6. Statistical Analysis
3. Results
3.1. Pharyngeal Pressure Measurements
3.2. Benchmark Surfactant Aerosol Deposition Studies
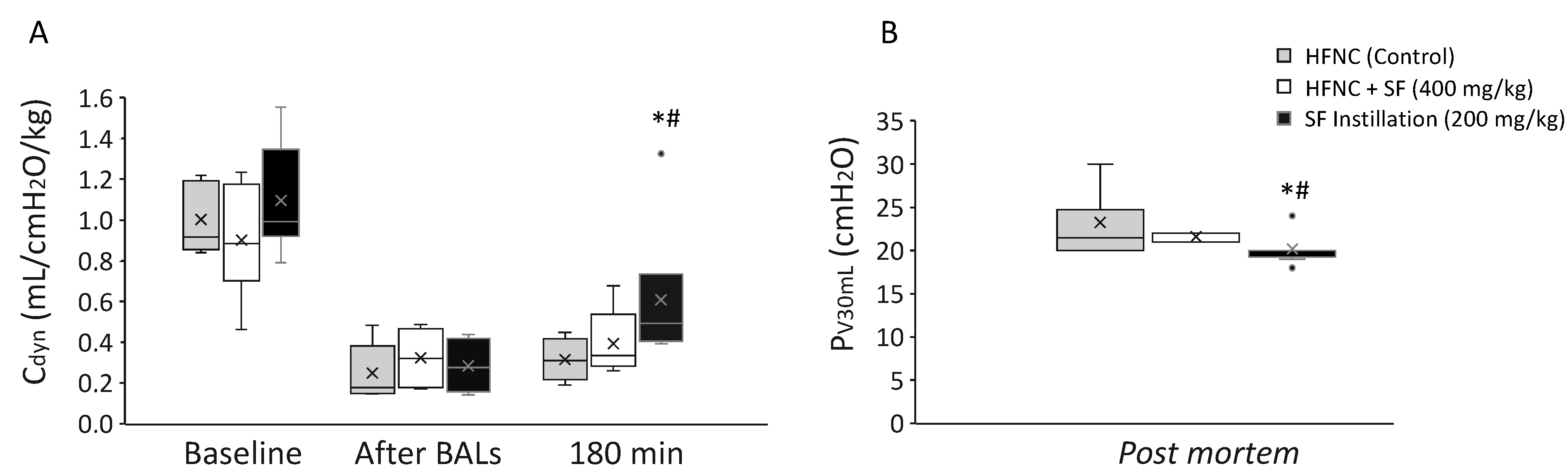
3.3. In Vivo Effect of Nebulized Surfactant during HFNC with the OptiflowTM System in Surfactant-Depleted Rabbits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schmölzer, G.M.; Kumar, M.; Pichler, G.; Aziz, K.; O’Reilly, M.; Cheung, P.-Y. Non-invasive versus invasive respiratory support in preterm infants at birth: Systematic review and meta-analysis. BMJ Br. Med. J. 2013, 347, f5980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, C.T.; Owen, L.S.; Manley, B.J.; Frøisland, D.H.; Donath, S.M.; Dalziel, K.M.; Pritchard, M.A.; Cartwright, D.W.; Collins, C.L.; Malhotra, A.; et al. Nasal High-Flow Therapy for Primary Respiratory Support in Preterm Infants. N. Engl. J. Med. 2016, 375, 1142–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.; Park, K.; Lee, E.H.; Choi, B.M. Humidified high flow nasal cannula versus nasal continuous positive airway pressure as an initial respiratory support in preterm infants with respiratory distress: A randomized, controlled non-inferiority trial. J. Korean Med. Sci. 2017, 32, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Lavizzari, A.; Colnaghi, M.; Ciuffini, F.; Veneroni, C.; Musumeci, S.; Cortinovis, I.; Mosca, F. Heated, Humidified High-Flow Nasal Cannula vs. Nasal Continuous Positive Airway Pressure for Respiratory Distress Syndrome of Prematurity: A Randomized Clinical Noninferiority Trial. JAMA Pediatr. 2016. [Google Scholar] [CrossRef] [Green Version]
- Liew, Z.; Fenton, A.C.; Harigopal, S.; Gopalakaje, S.; Brodlie, M.; O’Brien, C.J. Physiological effects of high-flow nasal cannula therapy in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, F87–F93. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, D.J.; Andersen, C.C.; Smith, K.; Holberton, J. Pharyngeal pressure with high-flow nasal cannulae in premature infants. J. Perinatol. 2008, 28, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Dargaville, P.A.; Gerber, A.; Johansson, S.; De Paoli, A.G.; Kamlin, C.O.F.; Orsini, F.; Davis, P.G. Incidence and outcome of CPAP failure in preterm infants. Pediatrics 2016, 138, e20153985. [Google Scholar] [CrossRef] [Green Version]
- Dargaville, P.A.; Aiyappan, A.; De Paoli, A.G.; Dalton, R.G.B.; Kuschel, C.A.; Kamlin, C.O.; Orsini, F.; Carlin, J.B.; Davis, P.G. Continuous Positive Airway Pressure Failure in Preterm Infants: Incidence, Predictors and Consequences. Neonatology 2013, 104, 8–14. [Google Scholar] [CrossRef]
- Lee, W.Y.; Choi, E.K.; Shin, J.; Lee, E.H.; Choi, B.M.; Hong, Y.S. Risk factors for treatment failure of heated humidified high-flow nasal cannula as an initial respiratory support in newborn infants with respiratory distress. Pediatr. Neonatol. 2020, 61, 174–179. [Google Scholar] [CrossRef]
- Raimondi, F.; de Winter, J.P.; De Luca, D. Lung ultrasound-guided surfactant administration: Time for a personalized, physiology-driven therapy. Eur. J. Pediatr. 2020, 179, 1909–1911. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Salomone, F.; Milesi, I.; Murgia, X.; Bonelli, S.; Pasini, E.; Dellacà, R.; Ventura, M.L.; Pillow, J. Aerosol drug delivery to spontaneously-breathing preterm neonates: Lessons learned. Respir. Res. 2021, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Ricci, F.; Catozzi, C.; Murgia, X.; Schlun, M.; Bucholski, A.; Hetzer, U.; Bonelli, S.; Lombardini, M.; Pasini, E.; et al. From bench to bedside: In vitro and in vivo evaluation of a neonate-focused nebulized surfactant delivery strategy. Respir. Res. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rey-Santano, C.; Mielgo, V.E.; Gomez-Solaetxe, M.A.; Bianco, F.; Salomone, F.; Loureiro, B. Nebulized Poractant alfa Reduces the Risk of Respiratory Failure at 72 Hours in Spontaneously Breathing Surfactant-Deficient Newborn Piglets. Crit. Care Med. 2020, 48, e523. [Google Scholar] [CrossRef]
- Rey-Santano, C.; Mielgo, V.; Gomez-Solaetxe, M.A.; Ricci, F.; Bianco, F.; Salomone, F.; Loureiro, B. Dose-Response Study on Surfactant Nebulization Therapy During Nasal Continuous Positive Airway Pressure Ventilation in Spontaneously Breathing Surfactant-Deficient Newborn Piglets *. Pediatr. Crit. Care Med. 2020, 21, e456. [Google Scholar] [CrossRef]
- Gregory, T.J.; Irshad, H.; Chand, R.; Kuehl, P.J. Deposition of Aerosolized Lucinactant in Nonhuman Primates. J. Aerosol Med. Pulm. Drug Deliv. 2019, 33, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Nord, A.; Linner, R.; Salomone, F.; Bianco, F.; Ricci, F.; Murgia, X.; Schlun, M.; Cunha-Goncalves, D.; Perez-de-Sa, V. Lung deposition of nebulized surfactant in newborn piglets: Nasal CPAP vs Nasal IPPV. Pediatr. Pulmonol. 2020, 55, 514–520. [Google Scholar] [CrossRef]
- Jorch, G.; Hartl, H.; Roth, B.; Kribs, A.; Gortner, L.; Schaible, T.; Hennecke, K.H.; Poets, C. To the editor: Surfactant aerosol treatment of respiratory distress syndrome in spontaneously breathing premature infants. Pediatr. Pulmonol. 1997, 24, 222–224. [Google Scholar] [CrossRef]
- Arroe, M.; Pedersen-Bjergaard, L.; Albertsen, P.; Bode, S.; Greison, G.; Jonsbo, F.; Arrøe, M.; Pedersen-Bjergaard, L.; Albertsen, P.; Bodé, S.; et al. Inhalation of aerosolized surfactant Exosurf to neonates treated with nasal continuous positive airway pressure. Prenat. Neonatal Med. 1998, 3, 346–352. [Google Scholar]
- Berggren, E.; Liljedahl, M.; Winbladh, B.; Andreasson, B.; Curstedt, T.; Robertson, B.; Schollin, J. Pilot study of nebulized surfactant therapy for neonatal respiratory distress syndrome. Acta Paediatr. 2000, 89, 460–464. [Google Scholar] [CrossRef]
- Mazela, J. Aerosol administration during nasal CPAP in newborns can be optimized. Respir. Care 2014, 59, 443–444. [Google Scholar] [CrossRef] [Green Version]
- Kamga Gninzeko, F.J.; Valentine, M.S.; Tho, C.K.; Chindal, S.R.; Boc, S.; Dhapare, S.; Momin, M.A.M.; Hassan, A.; Hindle, M.; Farkas, D.R.; et al. Excipient Enhanced Growth Aerosol Surfactant Replacement Therapy in an In Vivo Rat Lung Injury Model. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, G.; Iwatschenko, P.; Koch, W.; Windt, H.; Rast, M.; de Abreu, M.G.; Taut, F.J.H.; De Muynck, C. A Novel Continuous Powder Aerosolizer (CPA) for Inhalative Administration of Highly Concentrated Recombinant Surfactant Protein-C (rSP-C) Surfactant to Preterm Neonates. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 370–379. [Google Scholar] [CrossRef]
- Walther, F.J.; Gupta, M.; Lipp, M.M.; Chan, H.; Krzewick, J.; Gordon, L.M.; Waring, A.J. Aerosol delivery of dry powder synthetic lung surfactant to surfactant-deficient rabbits and preterm lambs on non-invasive respiratory support. Gates Open Res. 2019, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minocchieri, S.; Berry, C.A.; Pillow, J.J. Nebulised surfactant to reduce severity of respiratory distress: A blinded, parallel, randomised controlled trial. Arch. Dis. Child.-Fetal Neonatal Ed. 2018, 104, F313–F319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, J.J.; Gerday, E.; Minton, S.; Katheria, A.; Albert, G. Aerosolized Calfactant for Newborns With Respiratory Distress: A Randomized Trial. Pediatrics 2020, 146, e20193967. [Google Scholar] [CrossRef] [PubMed]
- Sood, B.G.; Thomas, R.; Delaney-Black, V.; Xin, Y.; Sharma, A.; Chen, X. Aerosolized Beractant in neonatal respiratory distress syndrome: A randomized fixed-dose parallel-arm phase II trial. Pulm. Pharmacol. Ther. 2021, 66, 10–16. [Google Scholar] [CrossRef]
- Bianco, F.; Pasini, E.; Nutini, M.; Murgia, X.; Stoeckl, C.; Schlun, M.; Hetzer, U.; Bonelli, S.; Lombardini, M.; Milesi, I.; et al. In vitro performance of an investigational vibrating-membrane nebulizer with surfactant under simulated, non-invasive neonatal ventilation conditions: Influence of continuous positive airway pressure interface and nebulizer positioning on the lung dose. Pharmaceutics 2020, 12, 257. [Google Scholar] [CrossRef] [Green Version]
- Ricci, F.; Casiraghi, C.; Storti, M.; D’Alò, F.; Catozzi, C.; Ciccimarra, R.; Ravanetti, F.; Cacchioli, A.; Villetti, G.; Civelli, M.; et al. Surfactant replacement therapy in combination with different non-invasive ventilation techniques in spontaneously-breathing, surfactant-depleted adult rabbits. PLoS ONE 2018, 13, e0200542. [Google Scholar] [CrossRef]
- Ricci, F.; Catozzi, C.; Murgia, X.; Rosa, B.; Amidani, D.; Lorenzini, L.; Bianco, F.; Rivetti, C.; Catinella, S.; Villetti, G.; et al. Physiological, biochemical, and biophysical characterization of the lung-lavaged spontaneously-breathing rabbit as a model for respiratory distress syndrome. PLoS ONE 2017, 12, e0169190. [Google Scholar] [CrossRef] [Green Version]
- Minocchieri, S.; Burren, J.M.; Bachmann, M.A.; Stern, G.; Wildhaber, J.; Buob, S.; Schindel, R.; Kraemer, R.; Frey, U.P.; Nelle, M. Development of the premature infant nose throat-model (PrINT-Model)—An upper airway replica of a premature neonate for the study of aerosol delivery. Pediatr. Res. 2008, 64, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, T.E.; Saville, A.; Adams, P.S.; Johnston, D.J.; Czachowski, M.R.; Domnina, Y.A.; Lin, J.-H.; Weiner, D.J.; Huber, A.S.; Sanchez De Toledo, J.; et al. Deposition studies of aerosol delivery by nasal cannula to infants. Pediatr. Pulmonol. 2019, 54, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Sunbul, F.S.; Fink, J.B.; Harwood, R.; Sheard, M.M.; Zimmerman, R.D.; Ari, A. Comparison of HFNC, bubble CPAP and SiPAP on aerosol delivery in neonates: An in-vitro study. Pediatr. Pulmonol. 2015, 50, 1099–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youngquist, T.M.; Richardson, C.P.; DiBlasi, R.M. Effects of condensate in the exhalation limb of neonatal circuits on airway pressure during bubble CPAP. Respir. Care 2013, 58, 1840–1846. [Google Scholar] [CrossRef] [Green Version]
- Réminiac, F.; Vecellio, L.; Loughlin, R.M.; Le Pennec, D.; Cabrera, M.; Vourc’h, N.H.; Fink, J.B.; Ehrmann, S. Nasal high flow nebulization in infants and toddlers: An in vitro and in vivo scintigraphic study. Pediatr. Pulmonol. 2017, 52, 337–344. [Google Scholar] [CrossRef]
- Janssens, H.M.; De Jongste, J.C.; Fokkens, W.J.; Robben, S.G.F.; Wouters, K.; Tiddens, H.A.W.M. The Sophia anatomical infant nose-throat (saint) model: A valuable tool to study aerosol deposition in infants. J. Aerosol Med. Depos. Clear. Eff. Lung 2001, 14, 433–441. [Google Scholar] [CrossRef]
- Bianco, F.; Pasini, E.; Nutini, M.; Murgia, X.; Stoeckl, C.; Schlun, M.; Hetzer, U.; Bonelli, S.; Lombardini, M.; Milesi, I.; et al. Extended pharmacopeial characterization of surfactant aerosols generated by a customized eflow neos nebulizer delivered through neonatal nasal prongs. Pharmaceutics 2020, 12, 319. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.F.; Ikegami, M.; Jobe, A.H.; Tabor, B. Aerosolized surfactant treatment of preterm lambs. J. Appl. Physiol. 1991, 70, 869–876. [Google Scholar] [CrossRef]
- Oei, J.L.; Kapadia, V.; Rabi, Y.; Saugstad, O.D.; Rook, D.; Vermeulen, M.J.; Boronat, N.; Thamrin, V.; Tarnow-Mordi, W.; Smyth, J.; et al. Neurodevelopmental outcomes of preterm infants after randomisation to initial resuscitation with lower (FiO2 < 0.3) or higher (FiO2 > 0.6) initial oxygen levels. An individual patient meta-analysis. Arch. Dis Child Fetal Neonatal Ed. 2021. [Google Scholar] [CrossRef]



| Surfactant Deposition (%) | Westmed (Infant Size) | Fisher & Paykel (Premature Size) |
|---|---|---|
| PrINT cast | 66.9 ± 5.9 | 46.2 ± 7.9 |
| Nebulizer + nasal cannula | 5.5 ± 0.3 | 18.8 ± 4.1 |
| Backup trap | 0.6 ± 0.1 | 4.2 ± 4.1 |
| Lung dose | 0.5 ± 0.45 | 1.8 ± 1.9 |
| Surfactant recovered vs. filled | 73.5 ± 6.0 | 71.0 ± 14.8 |
| Body Weight | Number of BALs | |
|---|---|---|
| HFNC (Control) | 1.80 ± 0.06 | 11.33 ± 1.56 |
| SF Instillation + HFNC | 1.88 ± 0.12 | 11.16 ± 1.56 |
| HFNC + neb SF (400 mg/kg) | 2.05 ± 0.12 | 9 ± 1.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, F.; Mersanne, A.; Storti, M.; Nutini, M.; Pellicelli, G.; Carini, A.; Milesi, I.; Lombardini, M.; Dellacà, R.L.; Thomson, M.A.; et al. Preclinical Assessment of Nebulized Surfactant Delivered through Neonatal High Flow Nasal Cannula Respiratory Support. Pharmaceutics 2022, 14, 1093. https://doi.org/10.3390/pharmaceutics14051093
Ricci F, Mersanne A, Storti M, Nutini M, Pellicelli G, Carini A, Milesi I, Lombardini M, Dellacà RL, Thomson MA, et al. Preclinical Assessment of Nebulized Surfactant Delivered through Neonatal High Flow Nasal Cannula Respiratory Support. Pharmaceutics. 2022; 14(5):1093. https://doi.org/10.3390/pharmaceutics14051093
Chicago/Turabian StyleRicci, Francesca, Arianna Mersanne, Matteo Storti, Marcello Nutini, Giulia Pellicelli, Angelo Carini, Ilaria Milesi, Marta Lombardini, Raffaele L. Dellacà, Merran A. Thomson, and et al. 2022. "Preclinical Assessment of Nebulized Surfactant Delivered through Neonatal High Flow Nasal Cannula Respiratory Support" Pharmaceutics 14, no. 5: 1093. https://doi.org/10.3390/pharmaceutics14051093
APA StyleRicci, F., Mersanne, A., Storti, M., Nutini, M., Pellicelli, G., Carini, A., Milesi, I., Lombardini, M., Dellacà, R. L., Thomson, M. A., Murgia, X., Lavizzari, A., Bianco, F., & Salomone, F. (2022). Preclinical Assessment of Nebulized Surfactant Delivered through Neonatal High Flow Nasal Cannula Respiratory Support. Pharmaceutics, 14(5), 1093. https://doi.org/10.3390/pharmaceutics14051093







