Functionalized Triblock Copolymers with Tapered Design for Anion Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Methods
- (1)
- The previous work [27] followed the framework by Sepehr et al. [15], which modeled two styrene monomers as the combination of one M (M for midblock) bead with two S (S for styrene block) beads. The revised version now models each styrene monomer as an S bead, which gives a more straightforward atom-to-bead mapping, making it easier to develop the force field parameters. The same choice is made by the Muller–Plathe group in modeling star-shaped polystyrene melts [50].
- (2)
- The previous work [25,27] used an iterative Boltzmann inversion (IBI)-style fitting method to obtain intramolecular force field parameters. A generic rule in an earlier work [45] is adopted where the bond stiffness and length are determined based on the coarse-grained size. On this basis, the harmonic bond stiffness K12 = 80 and K13 = 40, and the equilibrium bond length r12 = 0.8 and r13 = 1.6. The purpose of this rolling back is to develop a universal force field and explicitly monitor the effects of the tapered method.
- (3)
- This work slightly increases the molecular weight to 4378.4 g/mol of previous polymer models [27] to better explore the effects of the tapered method on membrane structures.
- (4)
- The repulsion parameter between S (polymer end blocks) and M (polymer midblock) is tuned up to 50, corresponding to the mismatch parameter of 25. This adjustment aims to reproduce the morphology of hydrated SEBS-Q reported in the literature. It should be noted that the mismatch between A and B for ABA triblock copolymers is usually chosen to c.a. 25 to reach the desired morphology [51,52]. More details are described in the beginning of Section 3.1
3. Results and Discussion
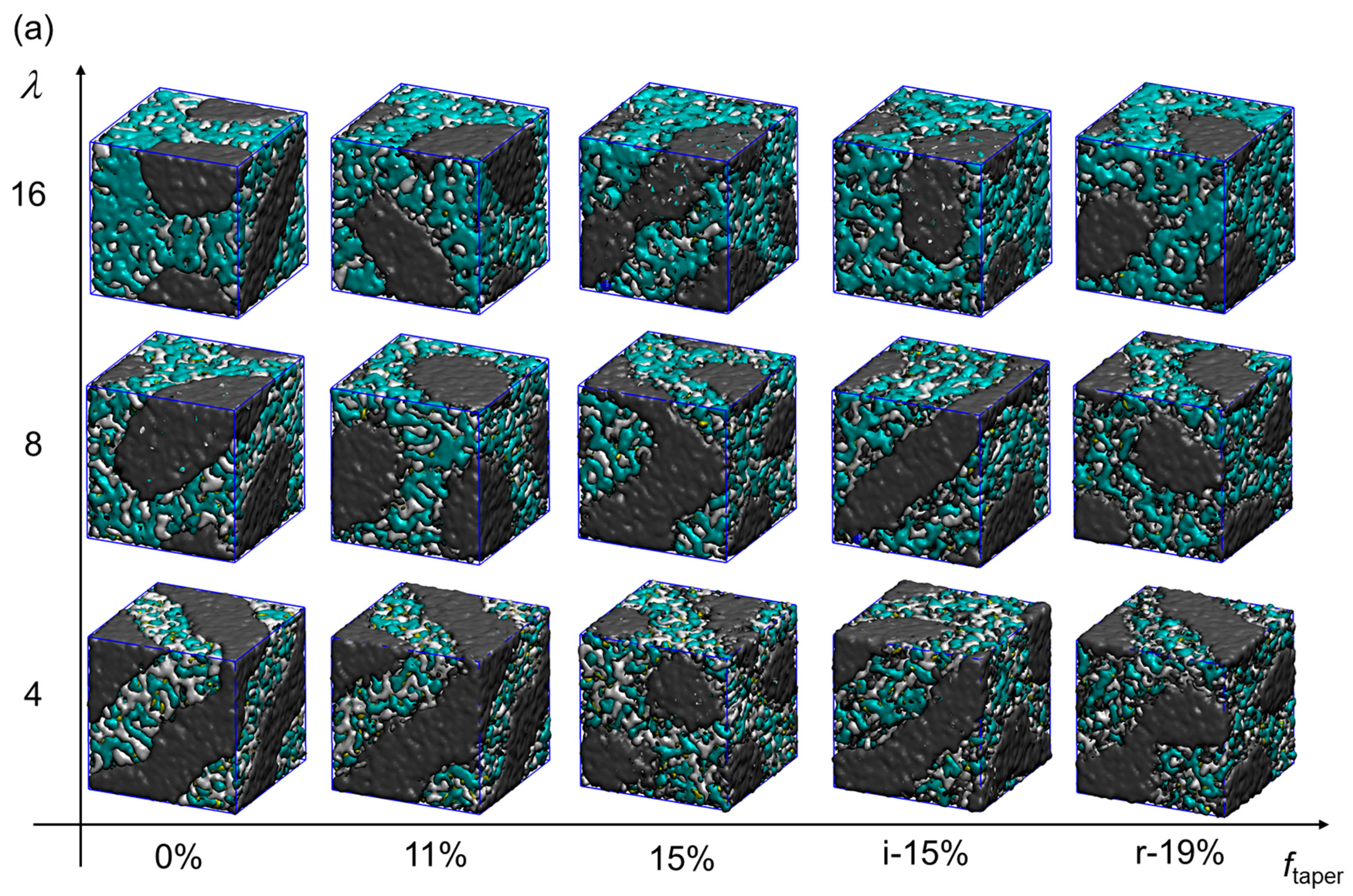
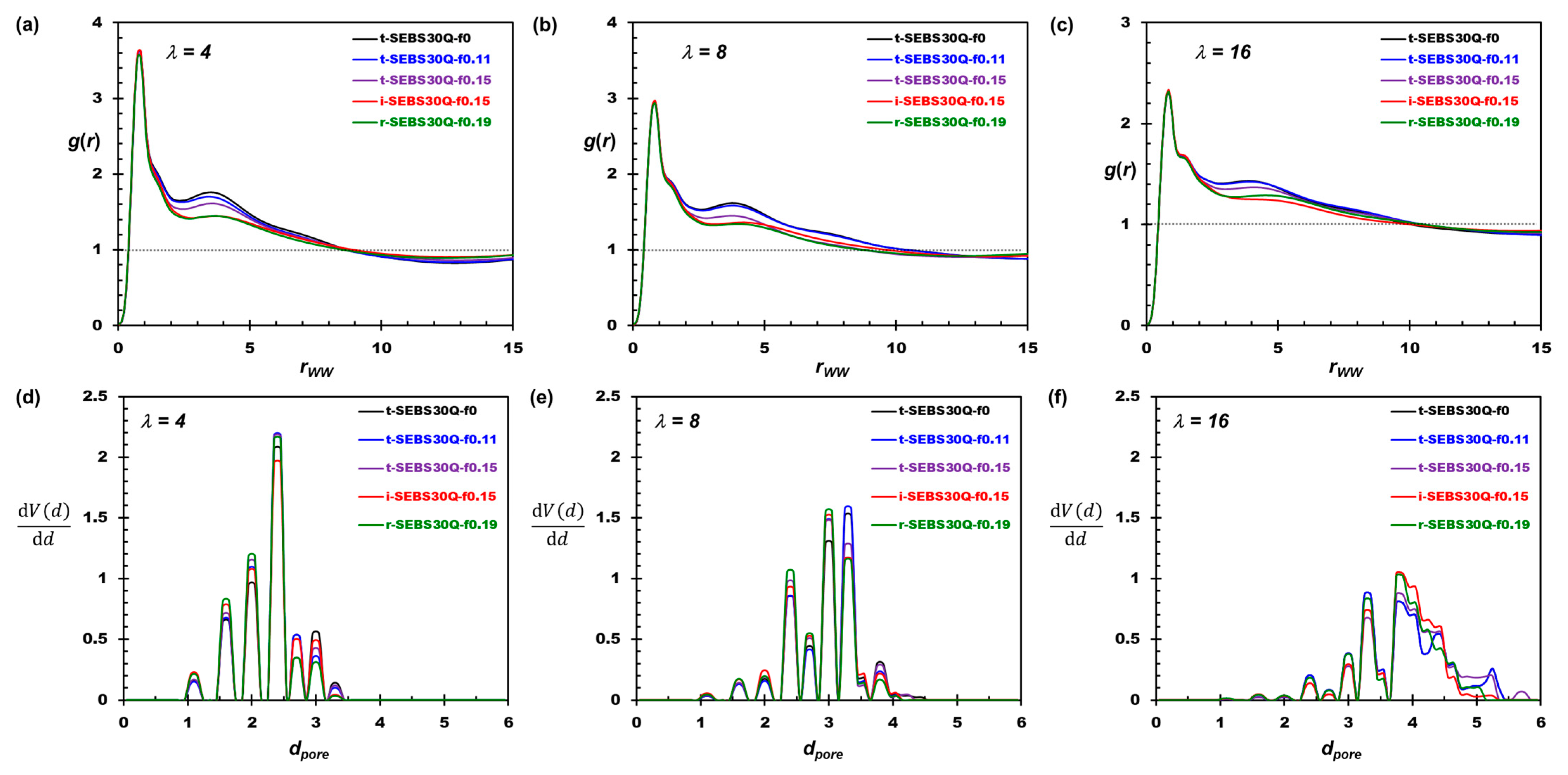
3.1. The Effects of Hydration and Tapering on Membrane Structure and Properties
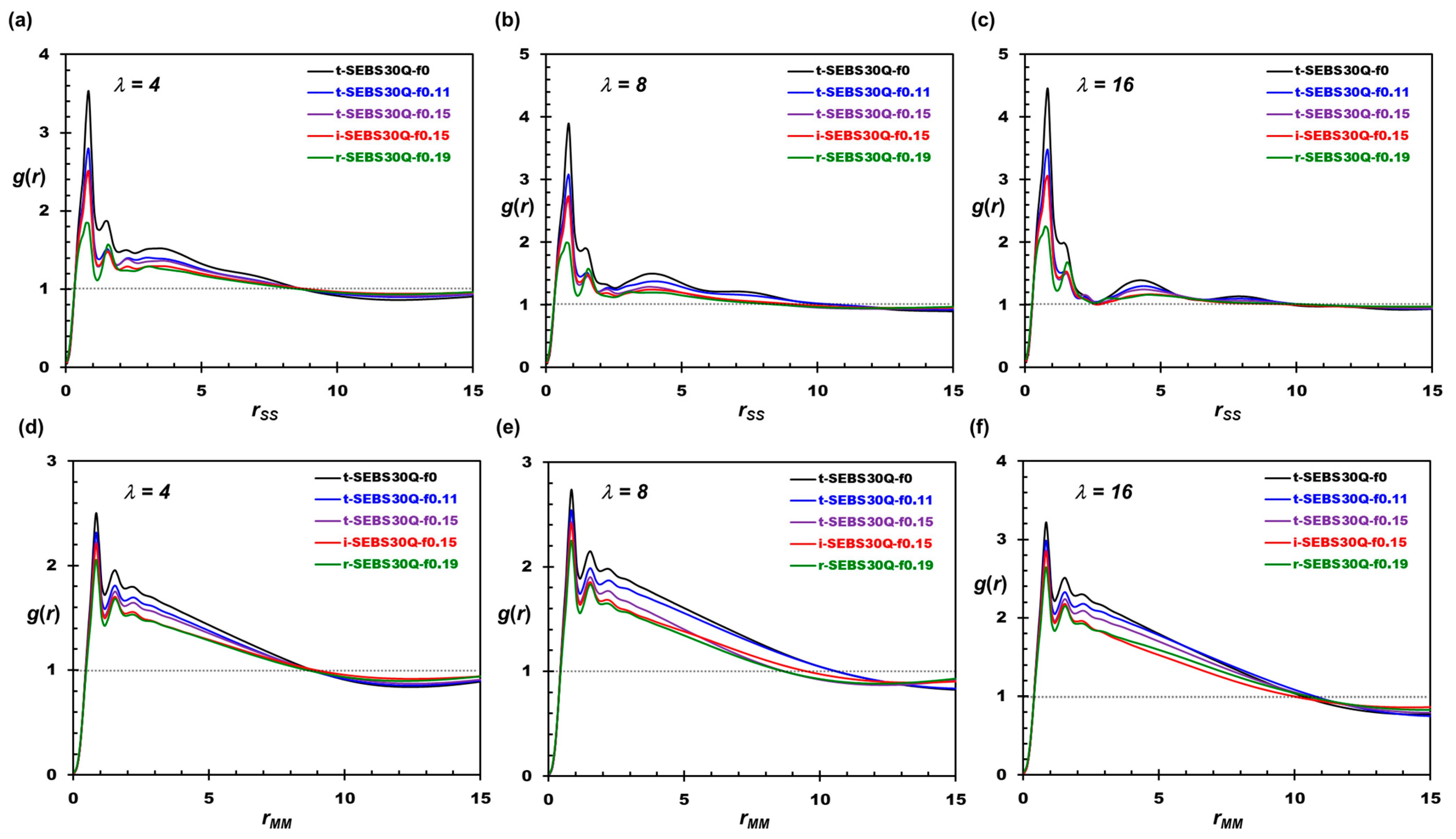
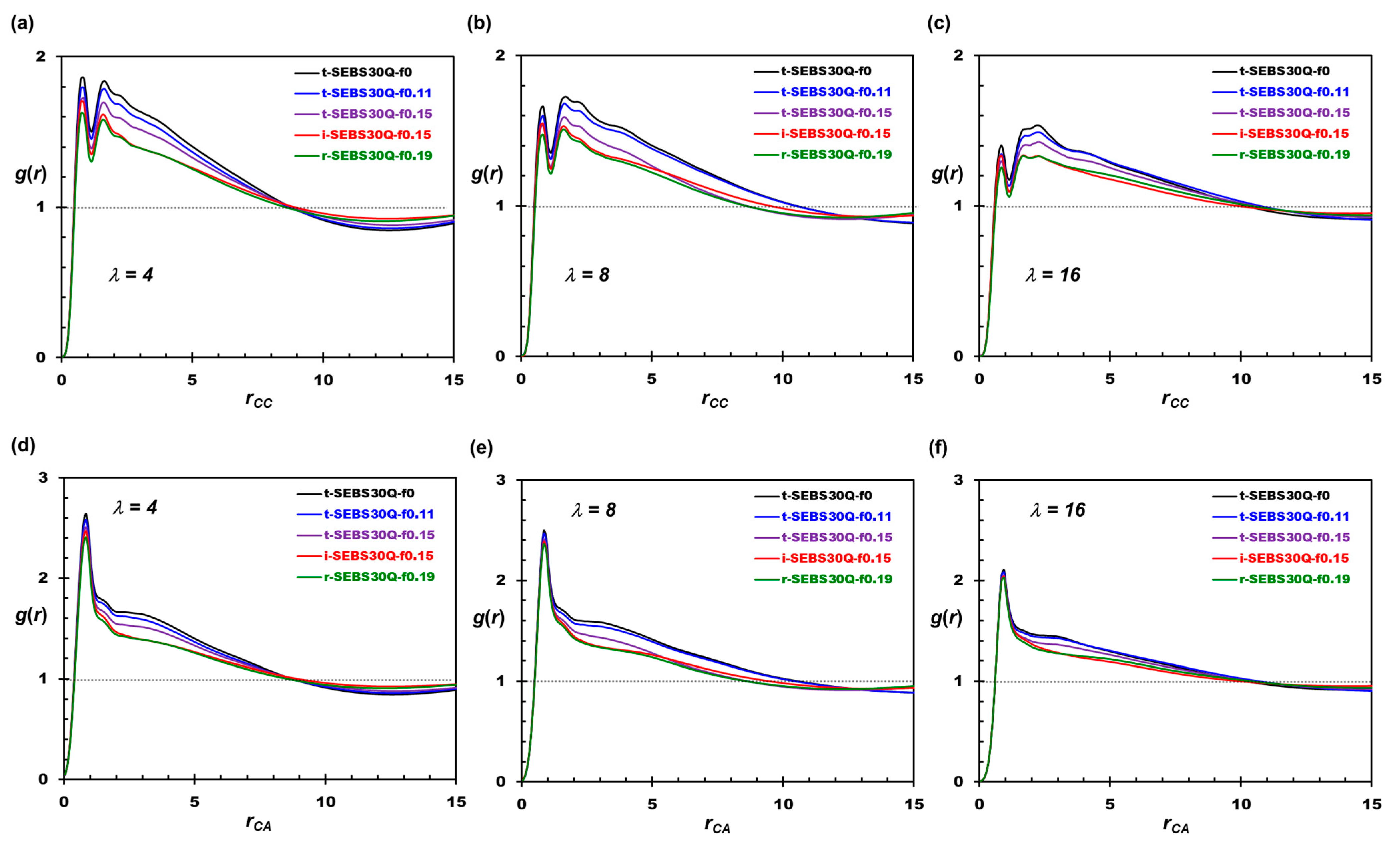
3.2. Tapering Effects on the Nanosegregation of Ionic Channels and Polymers
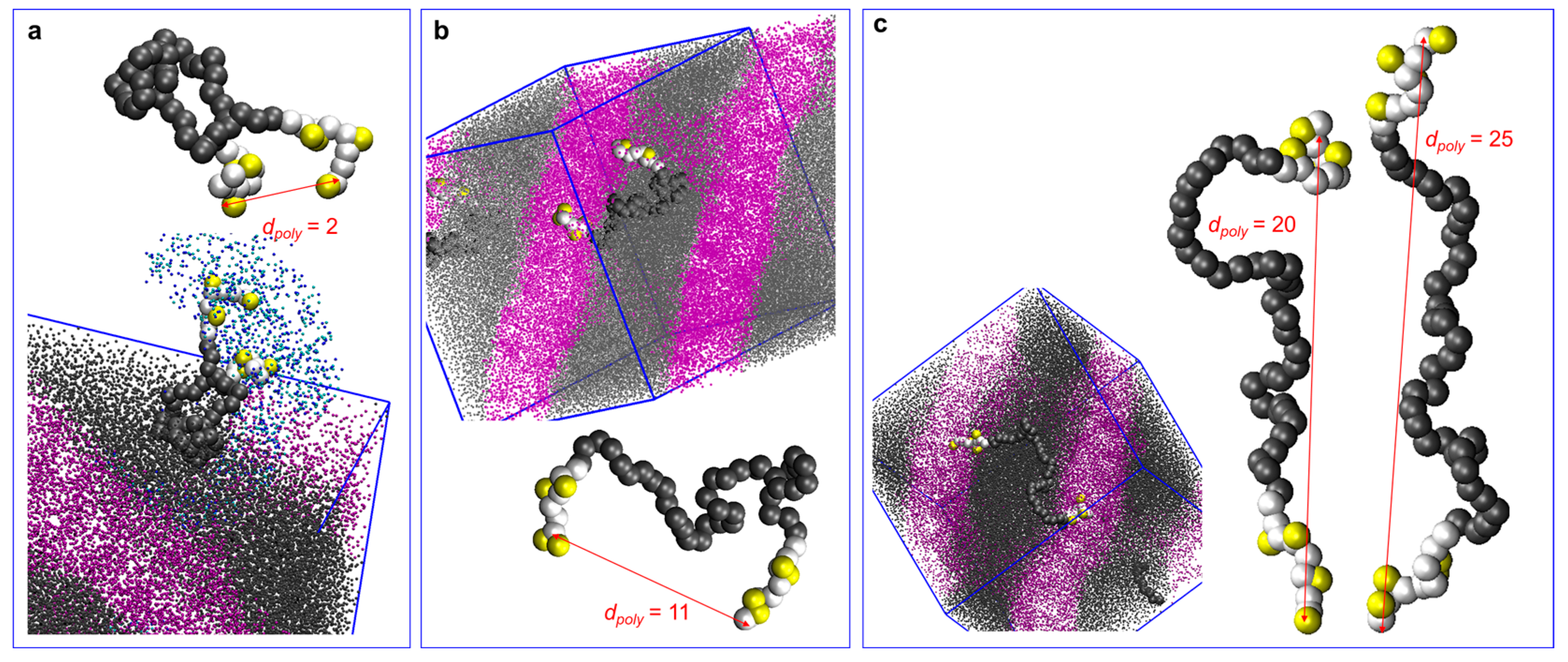
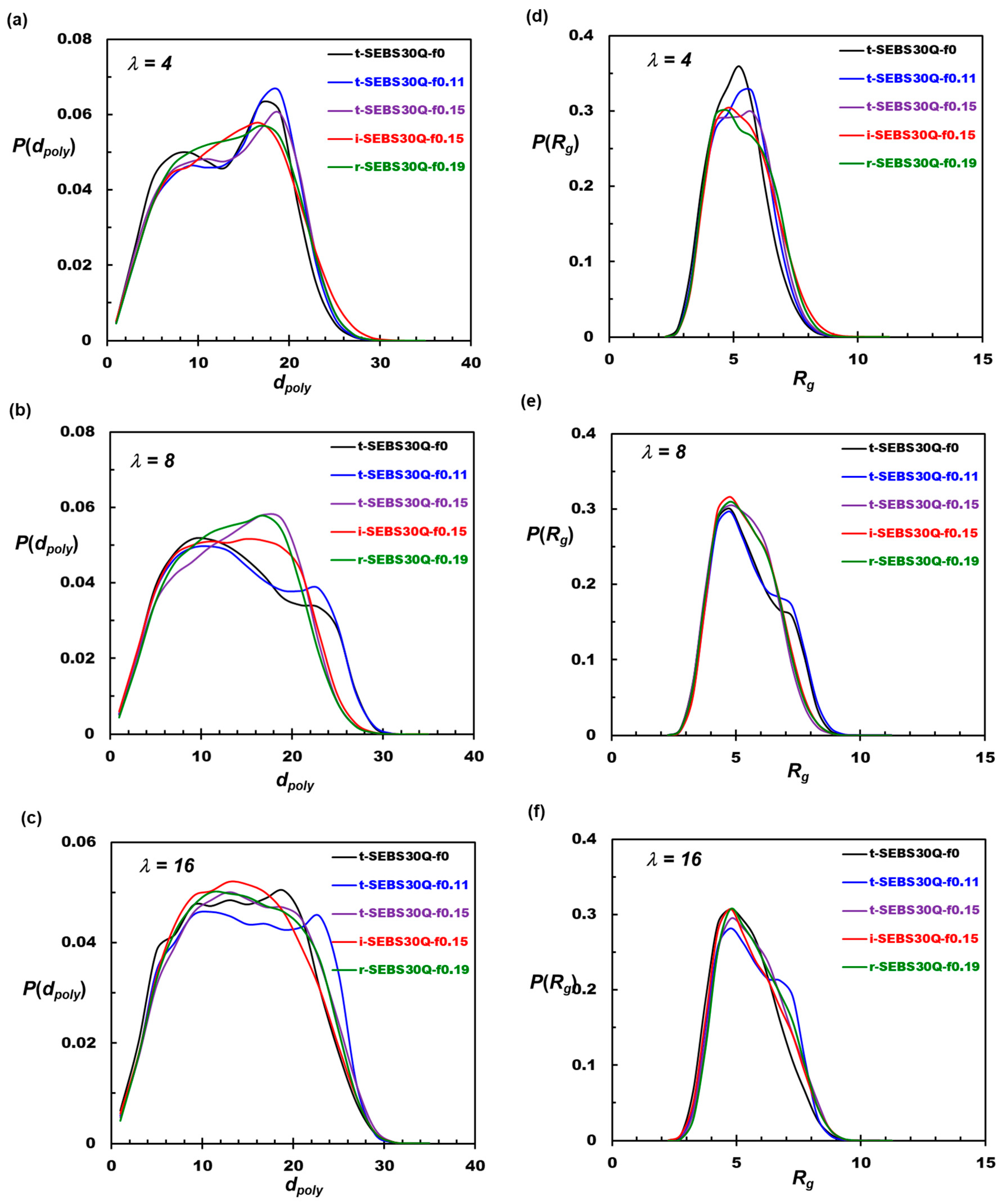
3.3. Tapering Effects on Polymer Conformations
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, E.J.; Jannaschet, P.; Miyatake, K.; Bae, C.; Noonan, K.; Fujimoto, C.; Holdcroft, S.; Varcoe, J.R.; Henkensmeier, D.; Guiver, M.D.; et al. Aryl ether-free polymer electrolytes for electrochemical and energy devices. Chem. Soc. Rev. 2024, 53, 5704–5780. [Google Scholar] [CrossRef]
- Song, W.; Zhang, X.; Yang, C.; Yang, Z.; Wu, L.; Ge, X.L.; Xu, T. Alkaline Membranes toward Electrochemical Energy Devices: Recent Development and Future Perspectives. ACS Cent. Sci. 2023, 9, 1538–1557. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.J.; Lee, Y.M. Anion-conducting polyelectrolytes for energy devices. Trends Chem. 2022, 4, 236–249. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, S.Y. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, A.M.; Kohl, A.P.; Kucernak, R.A.; Mustain, E.W.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, R.D.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Li, F.; Chan, S.H.; Tu, Z. Recent Development of Anion Exchange Membrane Fuel Cells and Performance Optimization Strategies: A Review. Chem. Rec. 2024, 24, e202300067. [Google Scholar] [CrossRef]
- You, W.; Noonan, K.J.T.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Elabd, Y.A. Ion transport in hydroxide conducting block copolymers. Mol. Syst. Des. Eng. 2019, 4, 519–530. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Ryu, C.Y.; Kim, S.Y.; Bae, C. Stable Elastomeric Anion Exchange Membranes Based on Quaternary Ammonium-Tethered Polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene Triblock Copolymers. Macromolecules 2015, 48, 7085–7095. [Google Scholar] [CrossRef]
- Dai, P.; Mo, Z.H.; Xu, R.W.; Zhang, S.; Wu, Y.X. Cross-Linked Quaternized Poly(styrene-b-(ethylene-co-butylene)-b-styrene) for Anion Exchange Membrane: Synthesis, Characterization and Properties. ACS Appl. Mater. Interfaces 2016, 8, 20329–20341. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Ma, W.; Zhao, S.; Xu, Z.; Sun, G. Highly stable poly(ethylene glycol)-grafted alkaline anion exchange membranes. J. Mater. Chem. A 2016, 4, 3886–3892. [Google Scholar] [CrossRef]
- Lin, C.X.; Wang, X.Q.; Hu, E.N.; Yang, Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.M. Quaternized triblock polymer anion exchange membranes with enhanced alkaline stability. J. Membr. Sci. 2017, 541, 358–366. [Google Scholar] [CrossRef]
- Sepehr, F.; Liu, H.; Luo, X.; Bae, C.; Tuckerman, M.E.; Hickner, M.A.; Paddison, S.J. Mesoscale Simulations of Anion Exchange Membranes Based on Quaternary Ammonium Tethered Triblock Copolymers. Macromolecules 2017, 50, 4397–4405. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Parrondo, J.; Ramani, V. Anion Exchange Membranes Based on Polystyrene-Block-Poly(ethylene-ran-butylene)-Block-Polystyrene Triblock Copolymers: Cation Stability and Fuel Cell Performance. J. Electrochem. Soc. 2017, 164, F1216–F1225. [Google Scholar] [CrossRef]
- Hao, J.K.; Gao, X.; Jiang, Y.; Zhang, H.; Luo, J.; Shao, Z.; Yi, B. Crosslinked high-performance anion exchange membranes based on poly (styrene-b-(ethylene-co-butylene)-b-styrene). J. Membr. Sci. 2018, 551, 66–75. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Park, S.; Han, J.; Maurya, S.; Mohanty, A.D.; Tian, D.; Saikia, N.; Hickner, M.A.; Ryu, C.Y.; Tuckerman, M.E.; et al. Synthesis of Aromatic Anion Exchange Membranes by Friedel-Crafts Bromoalkylation and Cross-Linking of Polystyrene Block Copolymers. Macromolecules 2019, 52, 2139–2147. [Google Scholar] [CrossRef]
- Luo, X.; Paddison, S.J. DPD simulations of anion exchange membrane: The effect of an alkyl spacer on the hydrated morphology. Solid State Ion. 2019, 339, 115012. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, X.; Paddison, S.J. DPD simulations of anion exchange membranes functionalized with various cationic groups and associated anions. Solid State Ion. 2019, 340, 115011. [Google Scholar] [CrossRef]
- Al Munsur, A.; Hossain, I.; Nam, S.Y.; Chae, J.E.; Kim, T.H. Hydrophobic-hydrophilic comb-type quaternary ammonium-functionalized SEBS copolymers for high performance anion exchange membranes. J. Membr. Sci. 2020, 599, 117829. [Google Scholar] [CrossRef]
- Al Munsur, A.; Hossain, I.; Nam, S.Y.; Chae, J.E.; Kim, T.H. Quaternary ammonium-functionalized hexyl bis(quaternary ammonium)-mediated partially crosslinked SEBSs as highly conductive and stable anion exchange membranes. Int. J. Hydrogen Energy 2020, 45, 15658–15671. [Google Scholar] [CrossRef]
- Gao, X.; Yu, H.M.; Xie, F.; Hao, J.; Shao, Z. High performance cross-linked anion exchange membrane based on aryl-ether free polymer backbones for anion exchange membrane fuel cell application. Sustain. Energy Fuels 2020, 4, 4057–4066. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Long, C.; Sang, J.; Tian, L.; Wang, F.; Wang, Z.; Zhu, H. Elastic and durable multi-cation-crosslinked anion exchange membrane based on poly(styrene-b-(ethylene-co-butylene)-b-styrene). J. Polym. Sci. 2020, 58, 2181–2196. [Google Scholar] [CrossRef]
- Lee, M.T. Designing Highly Conductive Block Copolymer-Based Anion Exchange Membranes by Mesoscale Simulations. J. Phys. Chem. B 2021, 125, 2729–2740. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Sang, J.; Cui, Y.; Zhu, H. Synthesis and characterization of a long side-chain double-cation crosslinked anion-exchange membrane based on poly(styrene-b-(ethylene-co-butylene)-b-styrene). Int. J. Hydrogen Energy 2021, 46, 36301–36313. [Google Scholar] [CrossRef]
- Chen, Q.G.; Lee, M.T. Anion Exchange Membranes for Fuel Cells Based on Quaternized Polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene Triblock Copolymers with Spacer-Sidechain Design. Polymers 2022, 14, 2860. [Google Scholar] [CrossRef]
- Han, J.; Liu, C.; Deng, C.; Zhang, Y.; Song, W.; Zheng, X.; Liu, X.; Zhang, Y.; Yang, X.; Ren, Z. Mechanically robust and highly conductive semi-interpenetrating network anion exchange membranes for fuel cell applications. J. Power Sources 2022, 548, 232097. [Google Scholar] [CrossRef]
- Liu, F.; Wahid, U.; Zhao, Z.; Liu, W.; Zhang, C. Design, synthesis and characterization of SEBS anion exchange membranes with ultrahigh dimensional stability. J. Polym. Res. 2022, 29, 343. [Google Scholar] [CrossRef]
- Sang, J.; Yang, L.; Li, Z.; Wang, F.; Wang, Z.; Zhu, H. Comb-shape d SEBS-base d anion exchange membranes with obvious microphase separation morphology. Electrochim. Acta 2022, 403, 139500. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Chen, N.; Lu, C.; Wang, F.; Zhu, H. Crosslinked poly (2,6-dimethyl-1,4-phenylene oxide) polyelectrolyte enhanced with poly (styrene-b-(ethylene-co-butylene)-b-styrene) for anion exchange membrane applications. J. Membr. Sci. 2018, 564, 492–500. [Google Scholar] [CrossRef]
- Rezayani, M.; Sharif, F.; Makki, H. Understanding ion diffusion in anion exchange membranes; effects of morphology and mobility of pendant cationic groups. J. Mater. Chem. A 2022, 10, 18295–18307. [Google Scholar] [CrossRef]
- Erimban, S.; Bombau, J.I.; Karnes, J.J.; Molinero, V. Degradation of Anion Exchange Membranes by Cation Elimination: Impact on Water Uptake, Nanostructure, and Ionic Mobility. J. Phys. Chem. C 2024, 128, 11033–11045. [Google Scholar] [CrossRef]
- Hodrokoukes, P.; Floudas, G.; Pispas, S.; Hadjichristidis, N. Microphase Separation in Normal and Inverse Tapered Block Copolymers of Polystyrene and Polyisoprene. 1. Phase State. Macromolecules 2001, 34, 650–657. [Google Scholar] [CrossRef]
- Kuan, W.; Roy, R.; Rong, L.; Hsiao, B.S.; Epps, T.H., III. Design and Synthesis of Network-Forming Triblock Copolymers Using Tapered Block Interfaces. ACS Macro Lett. 2012, 1, 519–523. [Google Scholar] [CrossRef]
- Kuan, W.F.; Remy, R.; Mackay, M.E.; Epps, T.H., III. Controlled ionic conductivity via tapered block polymer electrolytes. Rsc. Adv. 2015, 5, 12597–12604. [Google Scholar] [CrossRef]
- McIntosh, L.D.; Kubo, T.; Lodge, T.P. Morphology, Modulus, and Conductivity of a Triblock Terpolymer/Ionic Liquid Electrolyte Membrane. Macromolecules 2014, 47, 1090–1098. [Google Scholar] [CrossRef]
- Young, W.S.; Kuan, W.F.; Epps, T.H., III. Block Copolymer Electrolytes for Rechargeable Lithium Batteries. J. Polym. Sci. Part B-Polym. Phys. 2014, 52, 1–16. [Google Scholar] [CrossRef]
- Patterson, A.L.; Danielsen, S.P.O.; Yu, B.; Davidson, E.C.; Fredrickson, G.H.; Segalman, R.A. Sequence Effects on Block Copolymer Self-Assembly through Tuning Chain Conformation and Segregation Strength Utilizing Sequence-Defined Polypeptoids. Macromolecules 2019, 52, 1277–1286. [Google Scholar] [CrossRef]
- Patterson, A.L.; Danielsen, S.P.O.; Yu, B.; Davidson, E.C.; Fredrickson, G.H.; Segalman, R.A. Monomer Sequence Effects on Interfacial Width and Mixing in Self-Assembled Diblock Copolymers. Macromolecules 2020, 53, 3262–3272. [Google Scholar] [CrossRef]
- Steube, M.; Johann, T.; Barent, D.R.; Müller, A.H.E.; Frey, H. Rational design of tapered multiblock copolymers for thermoplastic elastomers. Prog. Polym. Sci. 2022, 124, 101488. [Google Scholar] [CrossRef]
- Gavrilov, A.A.; Potemkin, I.I. Copolymers with Nonblocky Sequences as Novel Materials with Finely Tuned Properties. J. Phys. Chem. B 2023, 127, 1479–1489. [Google Scholar] [CrossRef]
- Luo, M.; Brown, J.R.; Remy, R.A.; Scott, D.A.; Mackay, M.E.; Hall, L.M.; Epps, T.H., III. Determination of Interfacial Mixing in Tapered Block Polymer Thin Films: Experimental and Theoretical Investigations. Macromolecules 2016, 49, 5213–5222. [Google Scholar] [CrossRef]
- Sethuraman, V.; Ganesan, V. Segmental dynamics in lamellar phases of tapered copolymers. Soft Matter 2016, 12, 7818–7823. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Seo, Y.; Sides, S.W.; Hall, L.M. Unique Phase Behavior of Inverse Tapered Block Copolymers: Self Consistent Field Theory and Molecular Dynamics Simulations. Macromolecules 2017, 50, 5619–5626. [Google Scholar] [CrossRef]
- Seo, Y.; Sides, S.W.; Hall, L.M. Diffusion of Selective Penetrants in Interfacially Modified Block Copolymers from Molecular Dynamics Simulations. Acs Macro Lett. 2017, 6, 375–380. [Google Scholar] [CrossRef]
- Zhang, H.; Riggleman, R.A. Percolation of co-continuous domains in tapered copolymer networks. Mol. Syst. Des. Eng. 2023, 8, 115–122. [Google Scholar] [CrossRef]
- Español, P.; Warren, P.B. Perspective: Dissipative particle dynamics. J. Chem. Phys. 2017, 146. [Google Scholar] [CrossRef]
- Frenkel, D.; Smit, D. Understanding Molecular Simulation; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Wu, Z.H.; Muller-Plathe, F. Slip-Spring Hybrid Particle-Field Molecular Dynamics for Coarse-Graining Branched Polymer Melts: Polystyrene Melts as an Example. J. Chem. Theory Comput. 2022, 18, 3814–3828. [Google Scholar] [CrossRef]
- Droghetti, H.; Pagonabarraga, I.; Carbone, P.; Asinari, P.; Marchisio, M. Dissipative particle dynamics simulations of tri-block co-polymer and water: Phase diagram validation and microstructure identification. J. Chem. Phys. 2018, 149, 184903. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chang, H.Y.; Sheng, Y.J.; Tsao, H.K. Self-assembled polymersomes formed by symmetric, asymmetric and side-chain-tethered coil-rod-coil triblock copolymers. Soft Matt. 2014, 10, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.G.; Maczynski, A.; Goral, M.; Wisniewska-Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait-Pirson, M.C.; Hefter, G.T.; Kapuku, F.; et al. IUPAC-NIST solubility data series. 81. Hydrocarbons with water and seawater-revised and updated. Part 11. C-13-C-36 hydrocarbons with water. J. Phys. Chem. Ref. Data 2006, 35, 687–784. [Google Scholar] [CrossRef]
- Thomas, S.V. Handbook of Engineering and Speciality Thermoplastics: Polyethers and Polyesters; Thomas, S.V., Visakh, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2011; Volume 3. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Seaton, M.A.; Anderson, R.L.; Metz, s.; Smith, W. DL_MESO: Highly scalable mesoscale simulations. Mol. Simul. 2013, 39, 796–821. [Google Scholar] [CrossRef]
- Groot, R.D.; Rabone, K.L. Mesoscopic simulation of cell membrane damage, morphology change and rupture by nonionic surfactants. Biophys. J. 2001, 81, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Melchor, M.; Mayoral, E.; Velázquez, M.E.; Alejandre, J. Electrostatic interactions in dissipative particle dynamics using the Ewald sums. Biophys. J. 2006, 125, 224107. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, K.; Limpouchová, Z.; Štěpánek, M.; Šindelka, K.; Lísal, M. DPD Modelling of the Self- and Co-Assembly of Polymers and Polyelectrolytes in Aqueous Media: Impact on Polymer Science. Polymers 2022, 14, 404. [Google Scholar] [CrossRef]
- Heck, B.; Arends, P.; Ganter, M.; Kressler, J.; Stühn, B. SAXS and TEM Studies on Poly(styrene)-block-poly(ethene-co-but-1-ene)-block-poly(styrene) in Bulk and at Various Interfaces. Macromolecules 1997, 30, 4559–4566. [Google Scholar] [CrossRef]
- Lyubartsev, A.P.; Laaksonen, A.M. DynaMix–a scalable portable parallel MD simulation package for arbitrary molecular mixtures. Comput. Phys. Commun. 2000, 128, 565–589. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid lonomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Sarkisov, L.; Bueno-Perez, R.; Sutharson, M.; Fairen-Jimenez, D. Material Informatics with PoreBlazer v4.0 and CSD MOF Database. Chem. Mater. 2020, 32, 9849–9867. [Google Scholar] [CrossRef]
- Castaneda, S.; Ribadeneira, R. Description of Hydroxide Ion Structural Diffusion in a Quaternized SEBS Anion Exchange Membrane Using Ab Initio Molecular Dynamics. J. Phys. Chem. C 2020, 124, 9834–9851. [Google Scholar] [CrossRef]
- Dorenbos, G. Simulated and Experimental Trends Regarding Water Uptake in Polymeric Electrolyte Membranes. J. Phys. Chem. B 2023, 127, 9630–9641. [Google Scholar] [CrossRef]
- Allushi, A.; Bakvand, P.M.; Gong, H.; Jannasch, P. Hydroxide conducting BAB triblock copolymers tailored for durable high-performance anion exchange membranes. Mater. Adv. 2023, 4, 3733–3745. [Google Scholar] [CrossRef]


| Repulsion parameters | |||||
|---|---|---|---|---|---|
| aij | M | S | C | W | A |
| M | 25.0 | ||||
| S | 50.0 | 25.0 | |||
| C | 46.5 | 41.2 | 25.0 | ||
| W | 46.5 | 41.2 | 25.0 | 25.0 | |
| A | 25.0 | 25.0 | 25.0 | −8.3 | 25.0 |
| Bond parameters | |||||
| Harmoic | K(12) | r0(12) | K(13) | r0(13) | |
| 80.0 | 0.8 | 40.0 | 1.6 | ||
| Polymer. | λ | DW/DW_bulk | DA/DW_bulk | DA/DW | σ [mS/cm] | dmin(Rc) | dmax (Rc) |
|---|---|---|---|---|---|---|---|
| t-SEBS30Q-f0 | 4 | 0.27 | 0.50 | 1.85 | 18.7 | 2.1 | 3.3 |
| t-SEBS30Q-f0.11 | 4 | 0.26 | 0.51 | 1.97 | 18.8 | 2.1 | 3.3 |
| t-SEBS30Q-f0.15 | 4 | 0.25 | 0.52 | 2.08 | 19.4 | 2.1 | 3.3 |
| i-SEBS30Q-f0.15 | 4 | 0.23 | 0.49 | 2.11 | 18.3 | 2.1 | 3.3 |
| r-SEBS30Q-f0.19 | 4 | 0.23 | 0.51 | 2.22 | 19.0 | 2.1 | 3.3 |
| t-SEBS30Q-f0 | 8 | 0.33 | 0.56 | 1.69 | 18.8 | 2.8 | 4.5 |
| t-SEBS30Q-f0.11 | 8 | 0.34 | 0.56 | 1.64 | 18.8 | 2.8 | 4.1 |
| t-SEBS30Q-f0.15 | 8 | 0.32 | 0.56 | 1.74 | 18.8 | 2.8 | 4.3 |
| i-SEBS30Q-f0.15 | 8 | 0.31 | 0.51 | 1.65 | 17.2 | 2.8 | 4.1 |
| r-SEBS30Q-f0.19 | 8 | 0.30 | 0.55 | 1.81 | 18.5 | 2.8 | 4.1 |
| t-SEBS30Q-f0 | 16 | 0.93 | 1.65 | 1.77 | 45.7 | 3.6 | 5.4 |
| t-SEBS30Q-f0.11 | 16 | 0.84 | 1.70 | 2.02 | 47.3 | 3.6 | 5.7 |
| t-SEBS30Q-f0.15 | 16 | 0.90 | 1.59 | 1.78 | 44.3 | 3.6 | 5.4 |
| i-SEBS30Q-f0.15 | 16 | 0.99 | 1.67 | 1.68 | 46.3 | 3.6 | 5.2 |
| r-SEBS30Q-f0.19 | 16 | 0.84 | 1.50 | 1.78 | 41.8 | 3.6 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-T. Functionalized Triblock Copolymers with Tapered Design for Anion Exchange Membrane Fuel Cells. Polymers 2024, 16, 2382. https://doi.org/10.3390/polym16162382
Lee M-T. Functionalized Triblock Copolymers with Tapered Design for Anion Exchange Membrane Fuel Cells. Polymers. 2024; 16(16):2382. https://doi.org/10.3390/polym16162382
Chicago/Turabian StyleLee, Ming-Tsung. 2024. "Functionalized Triblock Copolymers with Tapered Design for Anion Exchange Membrane Fuel Cells" Polymers 16, no. 16: 2382. https://doi.org/10.3390/polym16162382






