A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring
Abstract
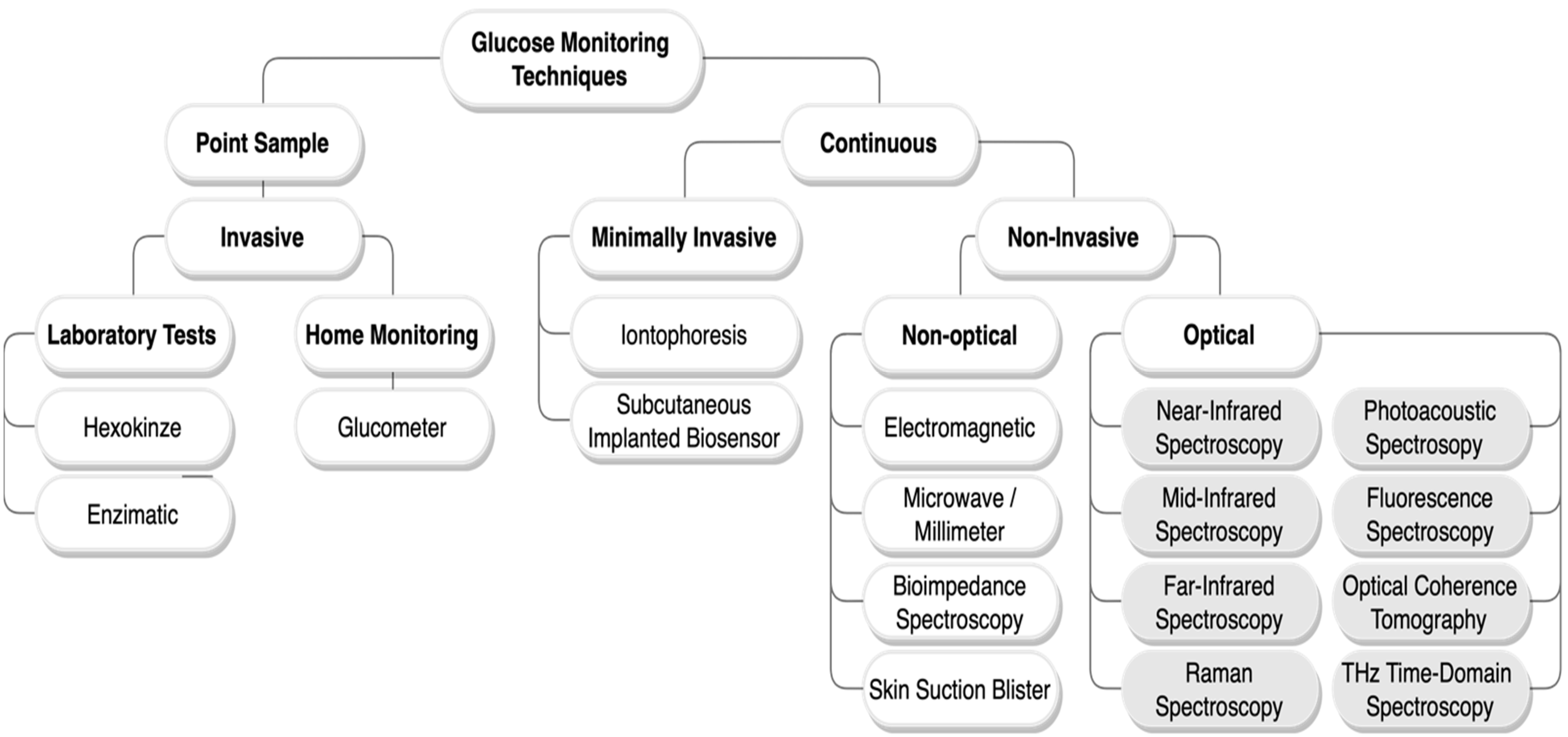
:1. Introduction
2. Non-Invasive Optical Techniques
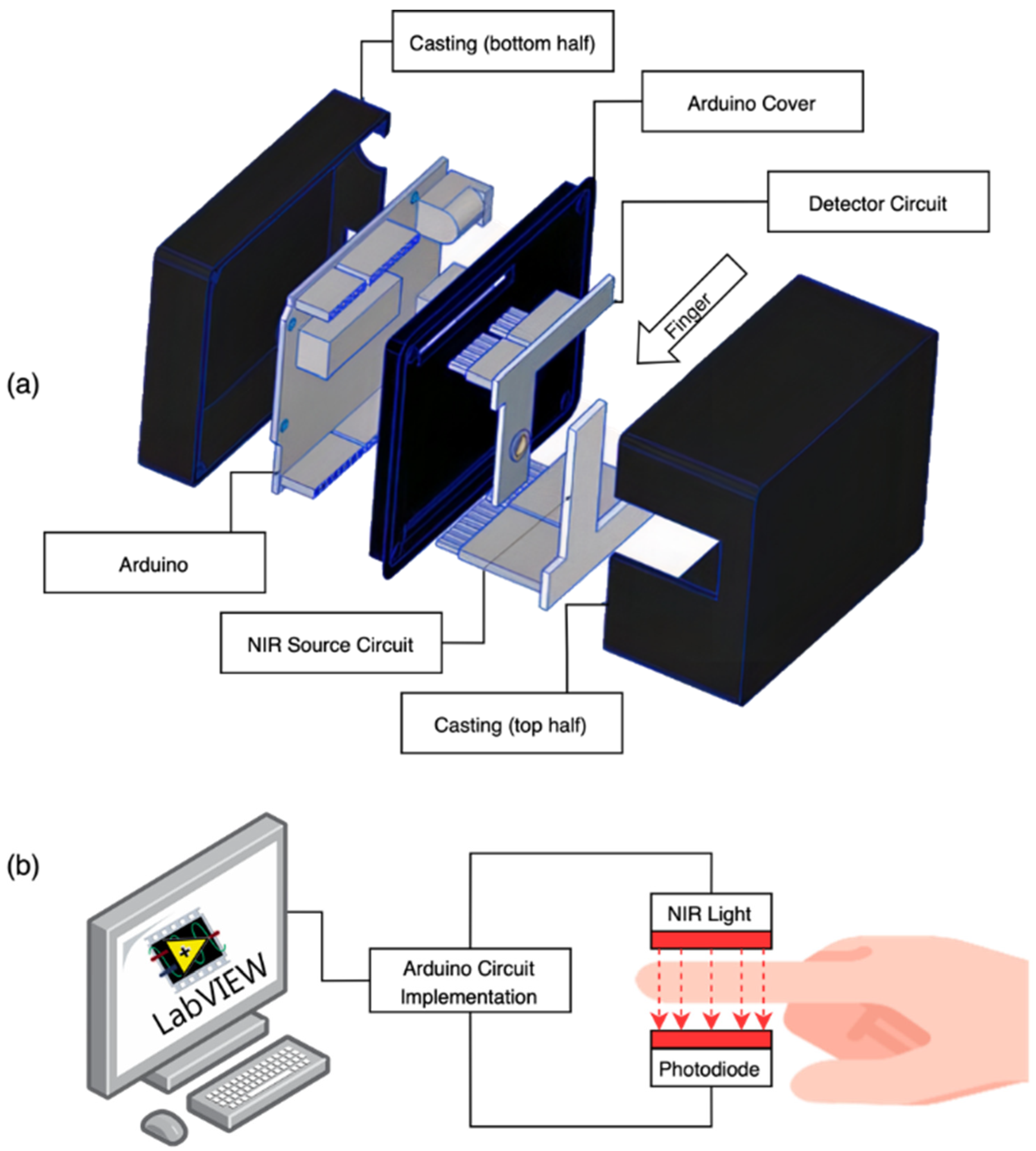
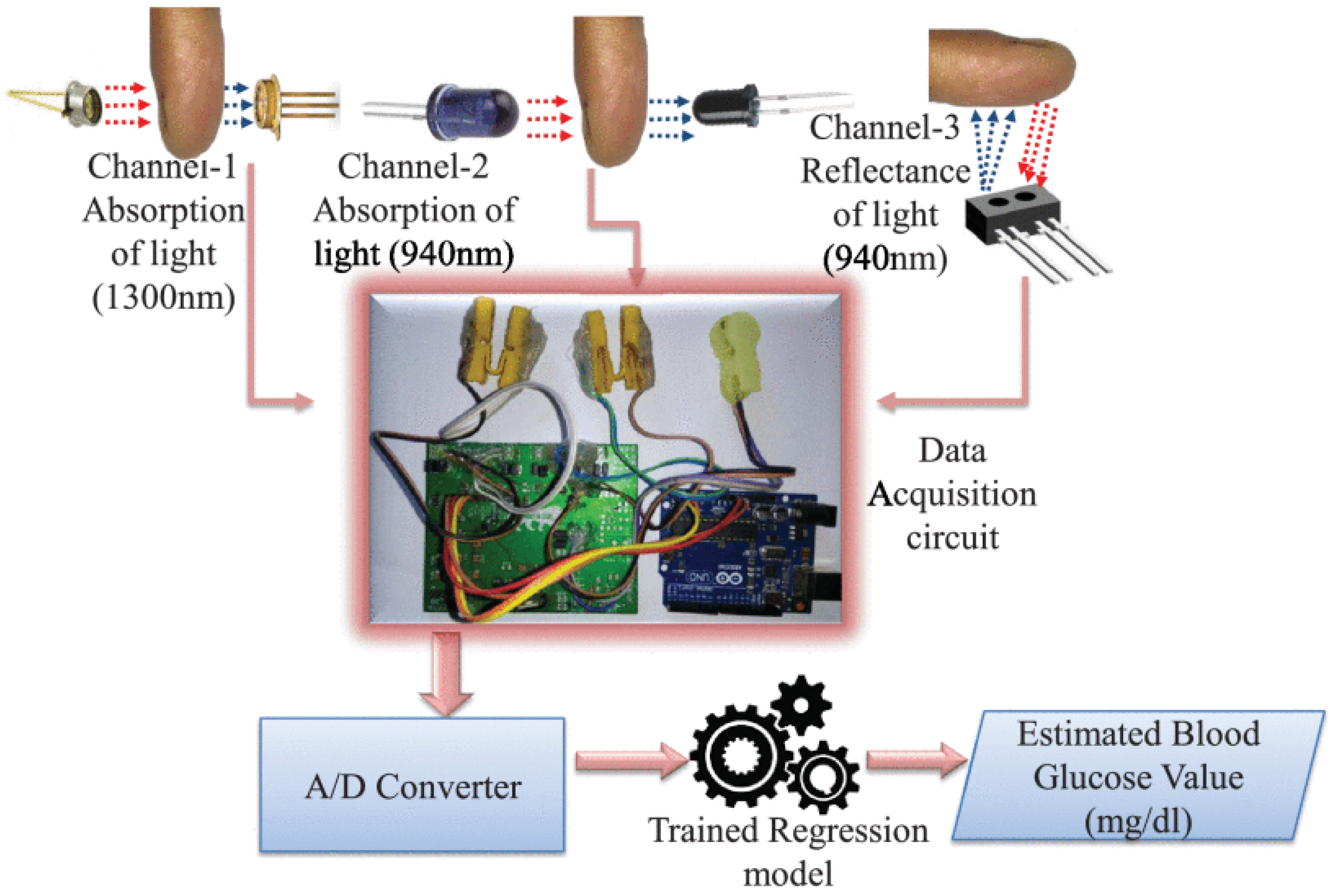
2.1. Near-Infrared (NIR) Spectroscopy
2.2. Mid-Infrared (MIR) Spectroscopy
2.3. Raman Spectroscopy
2.4. Far Infrared (FIR) Spectroscopy and Terahertz Time-Domain Spectroscopy (THz-TDS)
2.5. Fluorescent Spectroscopy
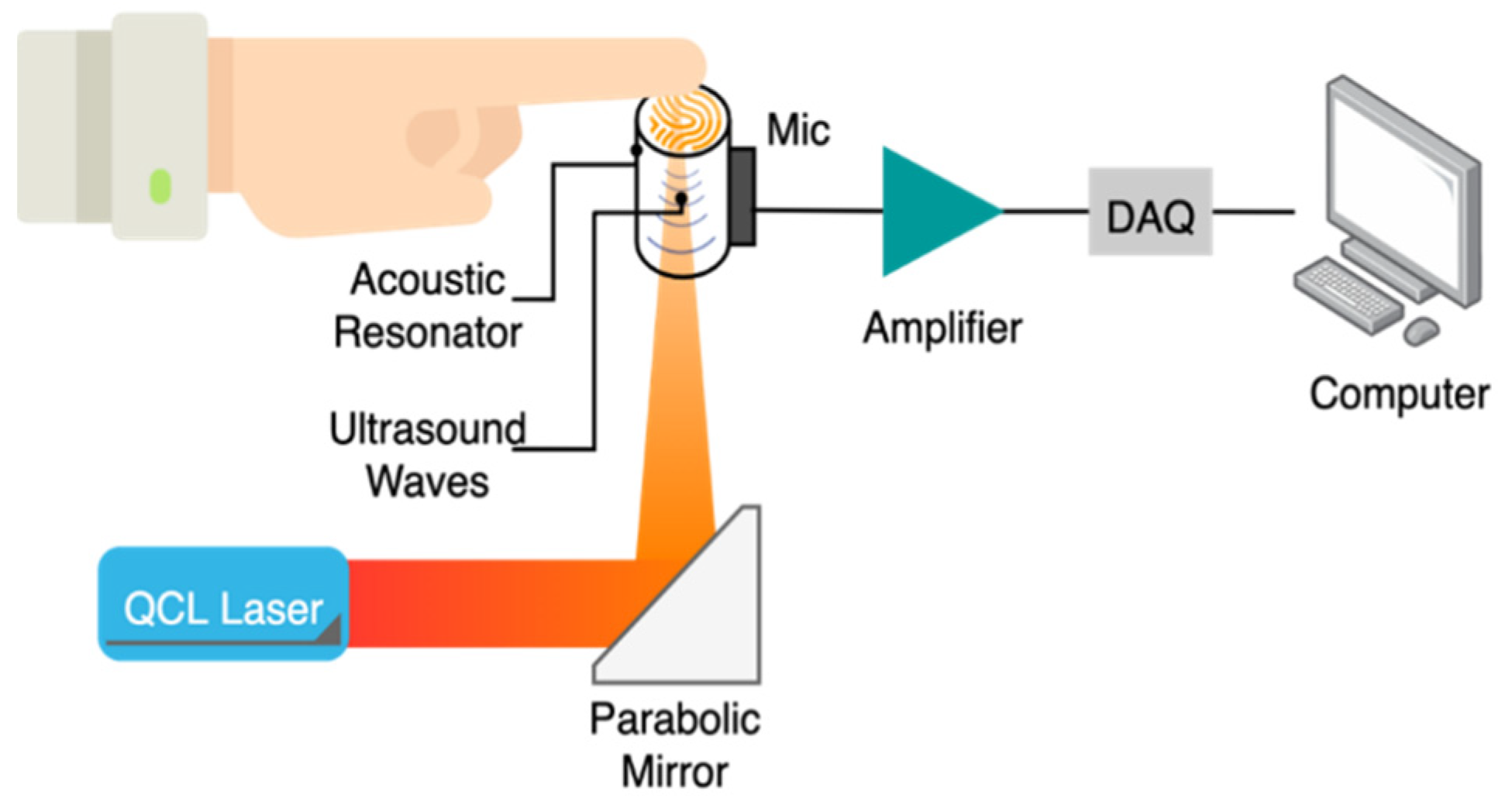
2.6. Photoacoustic Spectroscopy (PAS)
2.7. Optical Coherence Tomography (OCT)
3. Comparison
4. Regulations and Error Analysis
5. Commercial Devices
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerner, W.; Brückel, J. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2014, 122, 384–386. [Google Scholar] [CrossRef] [Green Version]
- Association, A.D. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [Green Version]
- Davis, B. What Is the Pathophysiology of Diabetes Mellitus? Available online: https://www.mvorganizing.org/what-is-the-pathophysiology-of-diabetes-mellitus/ (accessed on 6 October 2021).
- Mahler, R.J.; Adler, M.L. Type 2 Diabetes Mellitus: Update on Diagnosis, Pathophysiology, and Treatment. J. Clin. Endocrinol. Metab. 1999, 84, 1165–1171. [Google Scholar] [CrossRef]
- Gonzales, W.V.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [Green Version]
- Dinani, S.T.; Zekri, M.; Kamali, M. Regulation of blood glucose concentration in type 1 diabetics using single order sliding mode control combined with fuzzy on-line tunable gain, a simulation study. J. Med. Signals Sens. 2015, 5, 131–140. [Google Scholar] [CrossRef]
- Cryer, P.E. Minireview: Glucagon in the Pathogenesis of Hypoglycemia and Hyperglycemia in Diabetes. Endocrinology 2012, 153, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajjan, R.; Slattery, D.; Wright, E. Continuous Glucose Monitoring: A Brief Review for Primary Care Practitioners. Adv. Ther. 2019, 36, 579–596. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Daniels, J.; Liu, C.; Herrero, P.; Georgiou, P. Convolutional Recurrent Neural Networks for Glucose Prediction. IEEE J. Biomed. Health Inform. 2020, 24, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Jernelv, I.L.; Milenko, K.; Fuglerud, S.S.; Hjelme, D.R.; Ellingsen, R.; Aksnes, A. A review of optical methods for continuous glucose monitoring. Appl. Spectrosc. Rev. 2019, 54, 543–572. [Google Scholar] [CrossRef]
- Sim, J.; Ahn, C.; Jeong, E.; Kim, B. In vivo Microscopic Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring Invulnerable to Skin Secretion Products. Sci. Rep. 2018, 8, 1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokrekhodaei, M.; Quinones, S. Review of non-invasive glucose sensing techniques: Optical, electrical and breath acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Liu, S.; Jin, H.; Luo, Y.; Zheng, Z.; Gao, F.; Zheng, Y. Noninvasive Electromagnetic Wave Sensing of Glucose. Sensors 2019, 19, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delbeck, S.; Vahlsing, T.; Leonhardt, S.; Steiner, G.; Heise, H.M. Non-invasive monitoring of blood glucose using optical methods for skin spectroscopy—Opportunities and recent advances. Anal. Bioanal. Chem. 2019, 411, 63–77. [Google Scholar] [CrossRef]
- Maruo, K.; Yamada, Y. Near-infrared noninvasive blood glucose prediction without using multivariate analyses: Introduction of imaginary spectra due to scattering change in the skin. J. Biomed. Opt. 2015, 20, 047003. [Google Scholar] [CrossRef]
- Jeon, K.J.; Hwang, I.D.; Hahn, S.; Yoon, G. Comparison between transmittance and reflectance measurements in glucose determination using near infrared spectroscopy. J. Biomed. Opt. 2006, 11, 014022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, C. Research on non-invasive glucose concentration measurement by NIR transmission. In Proceedings of the 2015 IEEE International Conference on Computer and Communications, St. Louis, MO, USA, 23–27 March 2016; pp. 23–272016. [Google Scholar]
- Siesler, H.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy, Principles, Instruments, Applications; Wiley: Hoboken, NJ, USA, 2002; Volume 16, ISBN 978-3-527-30149-2. [Google Scholar]
- Yadav, J.; Rani, A.; Singh, V.; Mohan, B.; Murari, B.M. Prospects and limitations of non-invasive blood glucose monitoring using near-infrared spectroscopy. Biomed. Signal Process. Control 2015, 18, 214–227. [Google Scholar] [CrossRef]
- Rachim, V.P.; Chung, W.Y. Wearable-band type visible-near infrared optical biosensor for non-invasive blood glucose monitoring. Sens. Actuators B Chem. 2019, 286, 173–180. [Google Scholar] [CrossRef]
- Haxha, S.; Jhoja, J. Optical Based Noninvasive Glucose Monitoring Sensor Prototype. IEEE Photonics J. 2016, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Althobaiti, M.; Al-Naib, I. Optimization of Dual-Channel Near-Infrared Non-Invasive Glucose Level Measurement Sensors Based on Monte-Carlo Simulations. IEEE Photonics J. 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Joshi, A.M.; Jain, P.; Mohanty, S.P.; Agrawal, N. IGLU 2.0: A New Wearable for Accurate Non-Invasive Continuous Serum Glucose Measurement in IoMT Framework. IEEE Trans. Consum. Electron. 2020, 66, 327–335. [Google Scholar] [CrossRef]
- Kasahara, R.; Kino, S.; Soyama, S.; Matsuura, Y. Noninvasive glucose monitoring using mid-infrared absorption spectroscopy based on a few wavenumbers. Biomed. Opt. Express 2018, 9, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Salam, N.A.; Saad, W.H.M.; Manap, Z.; Salehuddin, F. The Evolution of Non-invasive Blood Glucose Monitoring System for Personal Application. J. Telecommun. Electron. Comput. Eng. 2016, 8, 59–65. [Google Scholar]
- Kumar, D.A.; Jayanthy, T. Review on Non-Invasive Blood Glucose Measurement Techniques. In Proceedings of the 2020 IEEE International Conference on Communication and Signal Processing, ICCSP 2020, Chennai, India, 28–30 July 2020; pp. 981–986. [Google Scholar]
- Kang, J.W.; Park, Y.S.; Chang, H.; Lee, W.; Singh, S.P.; Choi, W.; Galindo, L.H.; Dasari, R.R.; Nam, S.H.; Park, J.; et al. Direct observation of glucose fingerprint using in vivo Raman spectroscopy. Sci. Adv. 2020, 6, eaay5206. [Google Scholar] [CrossRef] [Green Version]
- Tonouchi, M. Cutting-edge terahertz technology. Nat. Photonics 2007, 1, 97–105. [Google Scholar] [CrossRef]
- Siegel, P.H. Terahertz technology. IEEE Trans. Microw. Theory Tech. 2002, 50, 910–928. [Google Scholar] [CrossRef]
- Siegel, P.H. Terahertz technology in biology and medicine. IEEE Trans. Microw. Theory Tech. 2004, 52, 2438–2447. [Google Scholar] [CrossRef]
- Jepsen, P.U.; Cooke, D.G.; Koch, M. Terahertz spectroscopy and imaging -Modern techniques and applications. Laser Photon. Rev. 2011, 5, 124–166. [Google Scholar] [CrossRef]
- Al-Naib, I. Biomedical Sensing with Conductively Coupled Terahertz Metamaterial Resonators. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 4700405. [Google Scholar] [CrossRef]
- D’Arco, A.; Di Fabrizio, M.; Dolci, V.; Petrarca, M.; Lupi, S. THz Pulsed Imaging in Biomedical Applications. Condens. Matter 2020, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; He, Y.; Liu, K.; Fan, S.; Parrott, E.P.J.; Pickwell-MacPherson, E. Recent advances in terahertz technology for biomedical applications. Quant. Imaging Med. Surg. 2017, 7, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Liakat, S.; Bors, K.A.; Xu, L.; Woods, C.M.; Doyle, J.; Gmachl, C.F. Noninvasive in vivo glucose sensing on human subjects using mid-infrared light. Biomed. Opt. Express 2014, 5, 2397. [Google Scholar] [CrossRef]
- Cherkasova, O.P.; Nazarov, M.M.; Shkurinov, A.P. Terahertz spectroscopy for diabetes diagnostics. EPJ Web Conf. 2017, 149, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Cherkasova, O.; Nazarov, M.; Shkurinov, A. Noninvasive blood glucose monitoring in the terahertz frequency range. Opt. Quantum Electron. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Gusev, S.I.; Soboleva, V.Y.; Kublanova, I.L.; Khodzitsky, M.K. Glucose level sensor based on metasurface in THz frequency range. AIP Conf. Proc. 2019, 2098, 020008. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Ma, S.; Wu, X.; Yang, W.; Zhang, W.; Li, X. Quantify Glucose Level in Freshly Diabetic’s Blood by Terahertz Time-Domain Spectroscopy. J. Infrared Millim. Terahertz Waves 2018, 39, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-K.; Kang, J.-H.; Lee, J.-S.; Kim, H.-S.; Kim, C.; Hun Kim, J.; Lee, T.; Son, J.-H.; Park, Q.-H.; Seo, M. Highly sensitive and selective sugar detection by terahertz nano-antennas. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Cardoso, G.G.; Rojas-Landeros, S.C.; Alfaro-Gomez, M.; Hernandez-Serrano, A.I.; Salas-Gutierrez, I.; Lemus-Bedolla, E.; Castillo-Guzman, A.R.; Lopez-Lemus, H.L.; Castro-Camus, E. Terahertz imaging for early screening of diabetic foot syndrome: A proof of concept. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, X.; Wang, Y.; Huang, G.; Yang, X.; Zhang, Y.; Xiong, Y.; Liu, L.; Zhao, X.; Fu, W. A novel THz molecule-selective sensing strategy in aqueous environments: THz-ATR spectroscopy integrated with a smart hydrogel. Talanta 2021, 228, 122213. [Google Scholar] [CrossRef] [PubMed]
- Aloraefy, M.; Pfefer, T.; Ramella-Roman, J.C.; Sapsford, K.E.; Joshua Pfefer, T.; Ramella-Roman, J.C.; Sapsford, K.E. In vitro evaluation of fluorescence glucose biosensor response. Sensors 2014, 14, 12127–12148. [Google Scholar] [CrossRef] [PubMed]
- Gamessa, T.W.; Suman, D.; Tadesse, Z.K. Blood glucose monitoring techniques: Recent advances, challenges and future perspectives. Int. J. Adv. Technol. Eng. Explor. 2018, 5, 335–344. [Google Scholar] [CrossRef]
- Nawaz, A.; Øhlckers, P.; Sælid, S.; Jacobsen, M.; Nadeem Akram, M. Review: Non-Invasive Continuous Blood Glucose Measurement Techniques. J. Bioinforma. Diabetes 2016, 1, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Pai, P.P.; Sanki, P.K.; Sahoo, S.K.; De, A.; Bhattacharya, S.; Banerjee, S. Cloud Computing-Based Non-Invasive Glucose Monitoring for Diabetic Care. IEEE Trans. Circuits Syst. Regul. Pap. 2018, 65, 663–676. [Google Scholar] [CrossRef]
- Izatt, J.A.; Choma, M.A.; Dhalla, A.H. Theory of optical coherence tomography. In Optical Coherence Tomography: Technology and Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Wojtkowski, M. High-speed optical coherence tomography: Basics and applications. Appl. Opt. 2010, 49, D30–D61. [Google Scholar] [CrossRef]
- Shelton, R.L.; Jung, W.; Sayegh, S.I.; Mccormick, D.T.; Kim, J.; Boppart, S.A. Optical coherence tomography for advanced screening in the primary care office. J. Biophotonics 2014, 7, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Mahdian, M.; Salehi, H.S.; Lurie, A.G.; Yadav, S.; Tadinada, A. Tissue characterization using optical coherence tomography and cone beam computed tomography: A comparative pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 98–103. [Google Scholar] [CrossRef]
- Nandy, S.; Sanders, M.; Zhu, Q. Classification and analysis of human ovarian tissue using full field optical coherence tomography. Biomed. Opt. Express 2016, 7, 5182–5187. [Google Scholar] [CrossRef] [Green Version]
- Nandy, S.; Salehi, H.S.; Wang, T.; Wang, X.; Sanders, M.; Kueck, A.; Brewer, M.; Zhu, Q. Correlating optical coherence elastography based strain measurements with collagen content of the human ovarian tissue. Biomed. Opt. Express 2015, 6, 3806–3811. [Google Scholar] [CrossRef] [Green Version]
- Esenaliev, R.O.; Larin, K.V.; Larina, I.V.; Motamedi, M. Noninvasive monitoring of glucose concentration with optical coherence tomography. Opt. Lett. 2001, 26, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Larin, K.V.; Eledrisi, M.S.; Motamedi, M.; Esenaliev, R.O. Noninvasive blood glucose monitoring with optical coherence tomography: A pilot study in human subjects. Diabetes Care 2002, 25, 2263–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larin, K.V.; Motamedi, M.; Ashitkov, T.V.; Esenaliev, R.O. Specificity of noninvasive blood glucose sensing using optical coherence tomography technique: A pilot study. Phys. Med. Biol. 2003, 48, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- De Pretto, L.R.; Yoshimura, T.M.; Ribeiro, M.S.; Zanardi de Freitas, A. Optical coherence tomography for blood glucose monitoring in vitro through spatial and temporal approaches. J. Biomed. Opt. 2016, 21, 086007. [Google Scholar] [CrossRef] [PubMed]
- Weatherbee, A.; Popov, I.; Vitkin, A. Accurate viscosity measurements of flowing aqueous glucose solutions with suspended scatterers using a dynamic light scattering approach with optical coherence tomography. J. Biomed. Opt. 2017, 22, 087033. [Google Scholar] [CrossRef]
- Phan, Q.-H.; Lo, Y.-L. Differential Mueller matrix polarimetry technique for non-invasive measurement of glucose concentration on human fingertip. Opt. Express 2017, 25, 15179. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-L.; Lo, Y.-L.; Liao, C.-C.; Phan, Q.-H. Noninvasive measurement of glucose concentration on human fingertip by optical coherence tomography. J. Biomed. Opt. 2018, 23, 047001. [Google Scholar] [CrossRef] [Green Version]
- Lundsgaard-Nielsen, S.M.; Pors, A.; Banke, S.O.; Henriksen, J.E.; Hepp, D.K.; Weber, A. Critical-depth Raman spectroscopy enables home-use non-invasive glucose monitoring. PLoS ONE 2018, 13, e0197134. [Google Scholar] [CrossRef] [Green Version]
- ISO—ISO 15197:2013—In Vitro Diagnostic Test Systems—Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. Available online: https://www.iso.org/standard/54976.html (accessed on 13 July 2021).
- Klonoff, D.C.; Lias, C.; Vigersky, R.; Clarke, W.; Parkes, J.L.; Sacks, D.B.; Kirkman, M.S.; Kovatchev, B. The surveillance error grid. J. Diabetes Sci. Technol. 2014, 8, 658–672. [Google Scholar] [CrossRef] [Green Version]
- Potts, R.O.; Tamada, J.A.; Tierney, M.J. Glucose monitoring by reverse iontophoresis. Diabetes. Metab. Res. Rev. 2002, 18, S49–S53. [Google Scholar] [CrossRef]
- Anand, P.K.; Shin, D.R.; Memon, M.L. Adaptive Boosting Based Personalized Glucose Monitoring System (PGMS) for Non-Invasive Blood Glucose Prediction with Improved Accuracy. Diagnostics 2020, 10, 285. [Google Scholar] [CrossRef]
- Self-Monitoring Blood Glucose Test Systems for Over-the-Counter Use|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/self-monitoring-blood-glucose-test-systems-over-counter-use (accessed on 13 July 2021).
- Ferrannini, E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: Problems and prospects. Endocr. Rev. 1998, 19, 477–490. [Google Scholar] [CrossRef]
- Bakker, G.J.; Vanbellinghen, M.C.; Scheithauer, T.P.; Verchere, C.B.; Stroes, E.S.; Timmers, N.K.L.M.; Herrema, H.; Nieuwdorp, M.; Verberne, H.J.; van Raalte, D.H. Pancreatic 18F-FDG uptake is increased in type 2 diabetes patients compared to non-diabetic controls. PLoS ONE 2019, 14, e0213202. [Google Scholar] [CrossRef]
- Lindholm, H.; Brolin, F.; Jonsson, C.; Jacobsson, H. The relation between the blood glucose level and the FDG uptake of tissues at normal PET examinations. EJNMMI Res. 2013, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfützner, A.; Strobl, S.; Demircik, F.; Redert, L.; Pfützner, J.; Pfützner, A.H.; Lier, A. Evaluation of a New Noninvasive Glucose Monitoring Device by Means of Standardized Meal Experiments. J. Diabetes Sci. Technol. 2018, 12, 1178–1183. [Google Scholar] [CrossRef]
- Segman, Y. (Joseph) Device and Method for Noninvasive Glucose Assessment. J. Diabetes Sci. Technol. 2018, 12, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cnoga. CoG—Hybrid Glucometer|Cnoga Digital Care. Available online: https://www.cnogacare.co/cog-hybrid-glucometer (accessed on 13 July 2021).
- Hadar, E.; Chen, R.; Toledano, Y.; Tenenbaum-Gavish, K.; Atzmon, Y.; Hod, H. Noninvasive, continuous, real-time glucose measurements compared to reference laboratory venous plasma glucose values. J. Matern. Fetal. Neonatal Med. 2018, 32, 3393–3400. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Verma, N.; Dungan, K. Monitoring Technologies-Continuous Glucose Monitoring, Mobile Technology, Biomarkers of Glycemic Control. Endotext 2020. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK279046/ (accessed on 10 October 2021).
- Gusev, M.; Poposka, L.; Spasevski, G.; Kostoska, M.; Koteska, B.; Simjanoska, M.; Ackovska, N.; Stojmenski, A.; Tasic, J.; Trontelj, J. Noninvasive Glucose Measurement Using Machine Learning and Neural Network Methods and Correlation with Heart Rate Variability. J. Sens. 2020, 2020, 9628281. [Google Scholar] [CrossRef]
- Lin, T. Non-Invasive Glucose Monitoring: A Review of Challenges and Recent Advances. Curr. Trends Biomed. Eng. Biosci. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Eversense Continuous Glucose Monitoring|Long-Term Continuous Glucose Monitor. Available online: https://www.eversensediabetes.com/ (accessed on 13 July 2021).
- Eversense Continuous Glucose Monitoring System—P160048/S006|FDA. Available online: https://www.fda.gov/medical-devices/recently-approved-devices/eversense-continuous-glucose-monitoring-system-p160048s006 (accessed on 13 July 2021).











| Author | Main Topics | Year | Ref. |
|---|---|---|---|
| Shokrekhodaei et al. | Review of non-invasive glucose measurements methods based on glucose intrinsic properties and blood/tissue characteristics to light, and respiration acetone analysis | 2020 | [15] |
| Zhang et al. | Review of non-invasive glucose measurement methods based on electromagnetic waves | 2019 | [16] |
| Villena Gonzales et al. | Review of invasive to minimally invasive and non-invasive glucose measurement methods, available commercial devices, accuracy assessment standards, the latest glucose sensors under development, and glucose estimation informatics. | 2019 | [5] |
| Jernelv et al. | Review of non-invasive continuous glucose measurement methods based on optical technologies, challenges facing these techniques, and current research status and prospects. | 2019 | [13] |
| Delbeck et al. | Overview of glucose measurement methods with a focus on Raman, mid-infrared, and near-infrared spectroscopies studies. Review of studies on photoplethysmography technology accompanies time resolution, wavelength dependence, and spatial resolution. | 2019 | [17] |
| Bruen et al. | Review of non-invasive continuous glucose measurement methods based on the estimation of glucose concentration in other physiological solutions rather than blood. | 2017 | [10] |
| Technology | Wavelength | Selectivity | Measurement Site | Merits | Drawbacks |
|---|---|---|---|---|---|
| NIR spectroscopy | 750–2500 nm | Good | Ear lobe, finger, forearm, cheek, lip mucosa, oral mucosa, and tongue |
|
|
| MIR spectroscopy | 2500–10,000 nm | Good, superior to NIR | Finger, skin, and oral mucosa |
|
|
| FIR spectroscopy | 10–1000 μm | Good | ISF |
|
|
| Raman spectroscopy | Visible light | Excellent | Eye, human skin |
|
|
| THz-TDS | 30 µm to 3 mm | Good | ISF |
|
|
| Fluorescence | Ultraviolet light, visible light | Excellent | Tears, human skin |
|
|
| PA spectroscopy | Ultraviolet light, NIR, and MIR | Good | Finger, forearm, and aqueous humor |
|
|
| Risk Zones | CEG Analysis | PEG Analysis |
|---|---|---|
| Zone A | Clinically valid treatment | No effect on clinical treatment |
| Zone B | Clinically uncritical treatment | Mild effect on clinical treatment |
| Zone C | Unnecessarily treatment | Possible to affect clinical treatment |
| Zone D | Dangerous fails to diagnose and treat | Serious medical risks |
| Zone E | Extremely dangerous lead to wrong treatment | Dangerous consequences |
| Agency | Glucose Level | Min. Accuracy | Ref. |
|---|---|---|---|
| U.S. Food and Drug Administration (FDA) | Full range | 95 ± 15% 99 ± 20% | [68] |
| European Medicines Agency (EMA) | ≥100 mg/dL | 95 ± 15% | [67] |
| Device | Company | Technology | Target Site | Accuracy | Approval | Ref. |
|---|---|---|---|---|---|---|
| TensorTip Combo Glucometer (CoG) | Cnoga Medical | VIS-NIR Spectroscopy Consists of four LEDs (625, 740, 850, 940 nm) and a color image sensor. | Fingertip | PEG: Zone A (96.6%) Zone B (3.4%) MARD: (14.4%) | CE approved FDA pending | [72,73,74] |
| Wizmi | Wear2b Ltd. | NIR Spectroscopy | Wrist | CEG: Zone A (93%) Zone B (7%) MARD: (7.23%) | Proof of concept | [75] |
| HELO Extense | World Global Network | NIR Spectroscopy | Fingertip | N/A | CE approved Discontinued | [5,76] |
| LTT | Light Touch Technology Ltd. | MIR Spectroscopy Uses a solid-state laser in the MIR region (6000–9000 nm) and optical parametric oscillation technology | Fingertip | N/A | Under development | [15] |
| NBM-200G | OrSense | Occlusion NIR Spectroscopy | Finger | CEG: Zone A (69.7%) Zone B (25.7%) MARD: (17.2%) | Discontinued | [77,78] |
| Eversense CGM | Senseonics | Fluorescence technology | Upper arm | MARD: (8.5%) | CE approved FDA approved | [79,80] |
| C8 MediSensors | Raman Spectroscopy | Abdomen | N/A | CE approved (Never released) | [78] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsunaidi, B.; Althobaiti, M.; Tamal, M.; Albaker, W.; Al-Naib, I. A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring. Sensors 2021, 21, 6820. https://doi.org/10.3390/s21206820
Alsunaidi B, Althobaiti M, Tamal M, Albaker W, Al-Naib I. A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring. Sensors. 2021; 21(20):6820. https://doi.org/10.3390/s21206820
Chicago/Turabian StyleAlsunaidi, Bushra, Murad Althobaiti, Mahbubunnabi Tamal, Waleed Albaker, and Ibraheem Al-Naib. 2021. "A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring" Sensors 21, no. 20: 6820. https://doi.org/10.3390/s21206820
APA StyleAlsunaidi, B., Althobaiti, M., Tamal, M., Albaker, W., & Al-Naib, I. (2021). A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring. Sensors, 21(20), 6820. https://doi.org/10.3390/s21206820







