Ion Hopping and Constrained Li Diffusion Pathways in the Superionic State of Antifluorite Li2O
Abstract
:1. Introduction
2. Simulation Methods
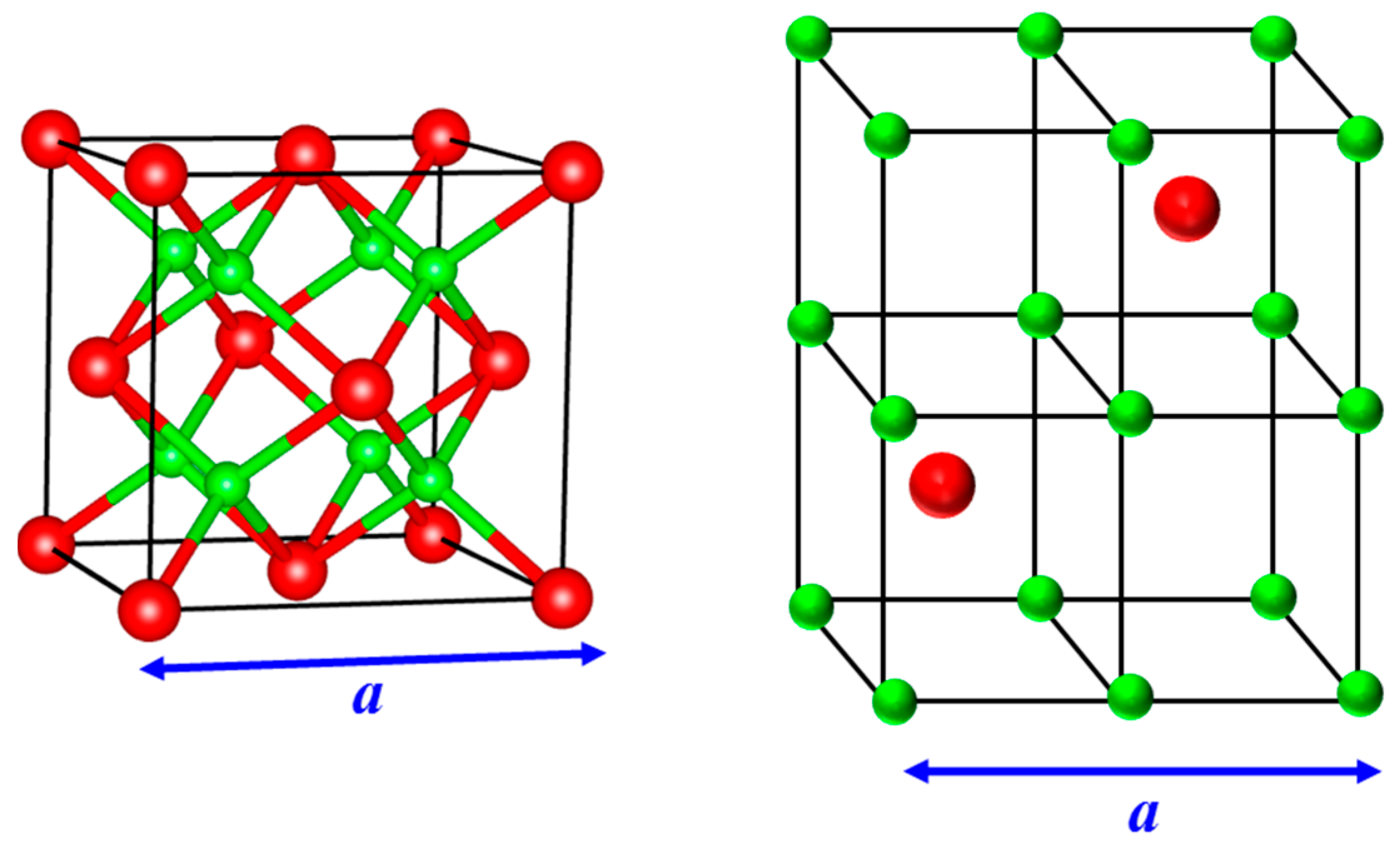
3. Results
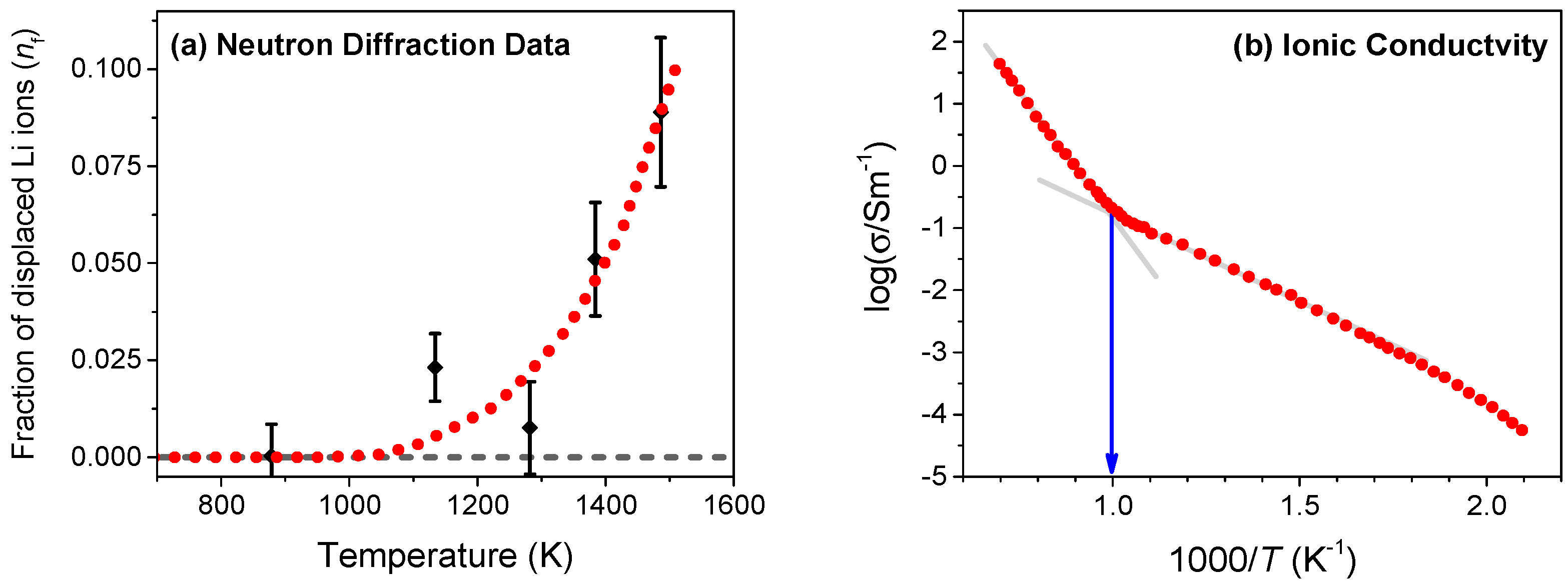
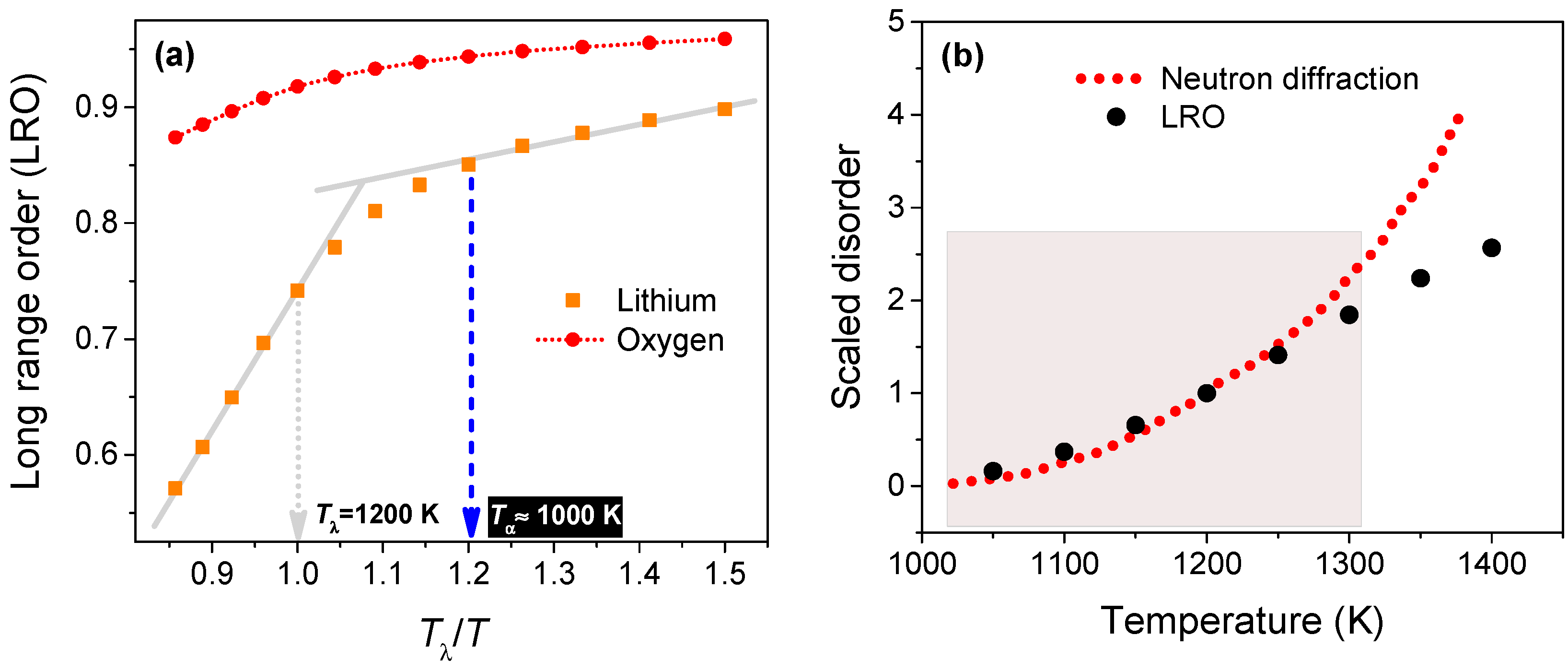
3.1. Onset of Disorder from Reported Experimental Data
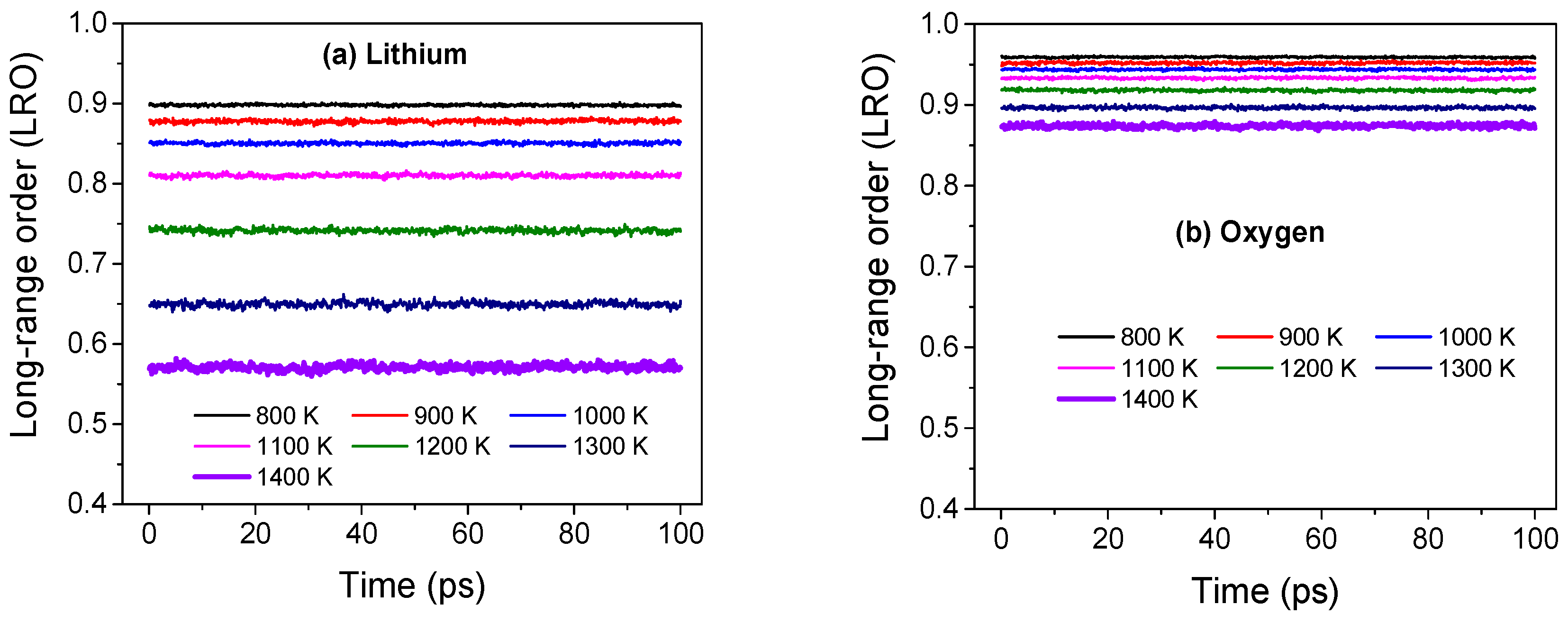
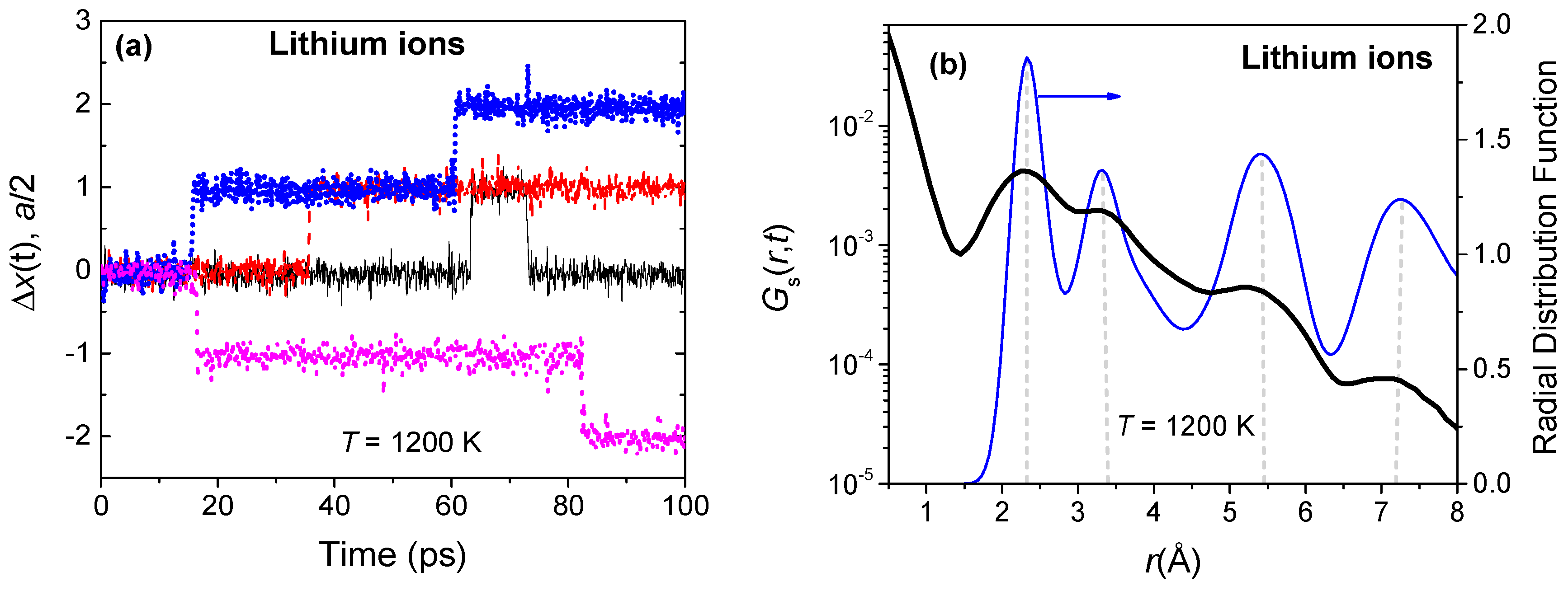
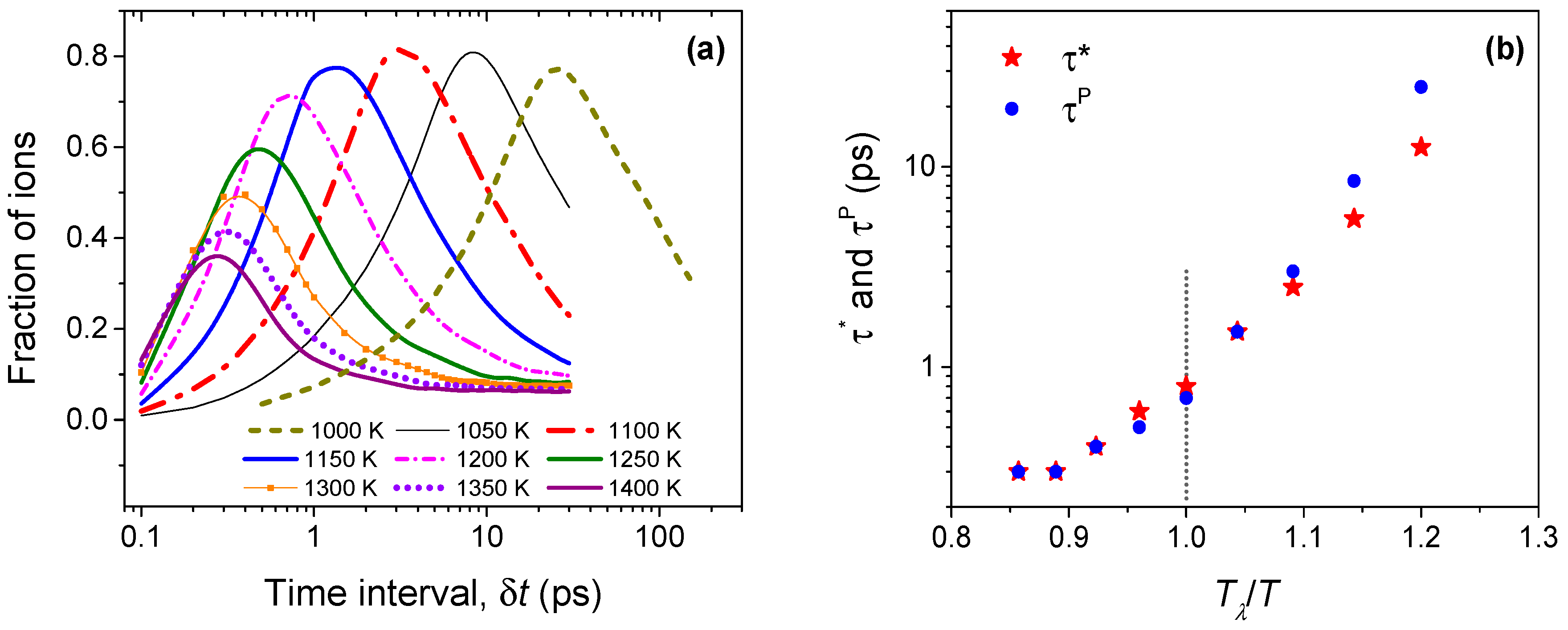
3.2. Hopping and Cooperativity among LI Ions
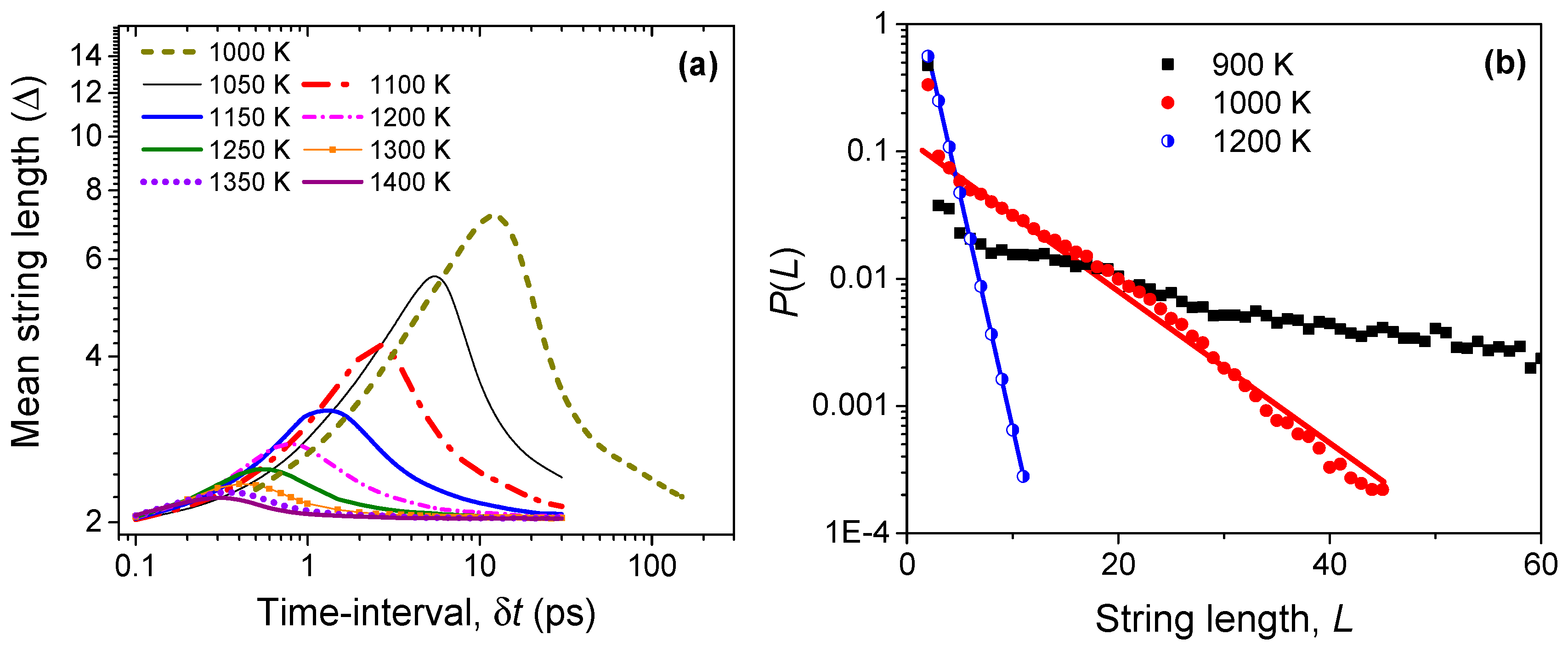
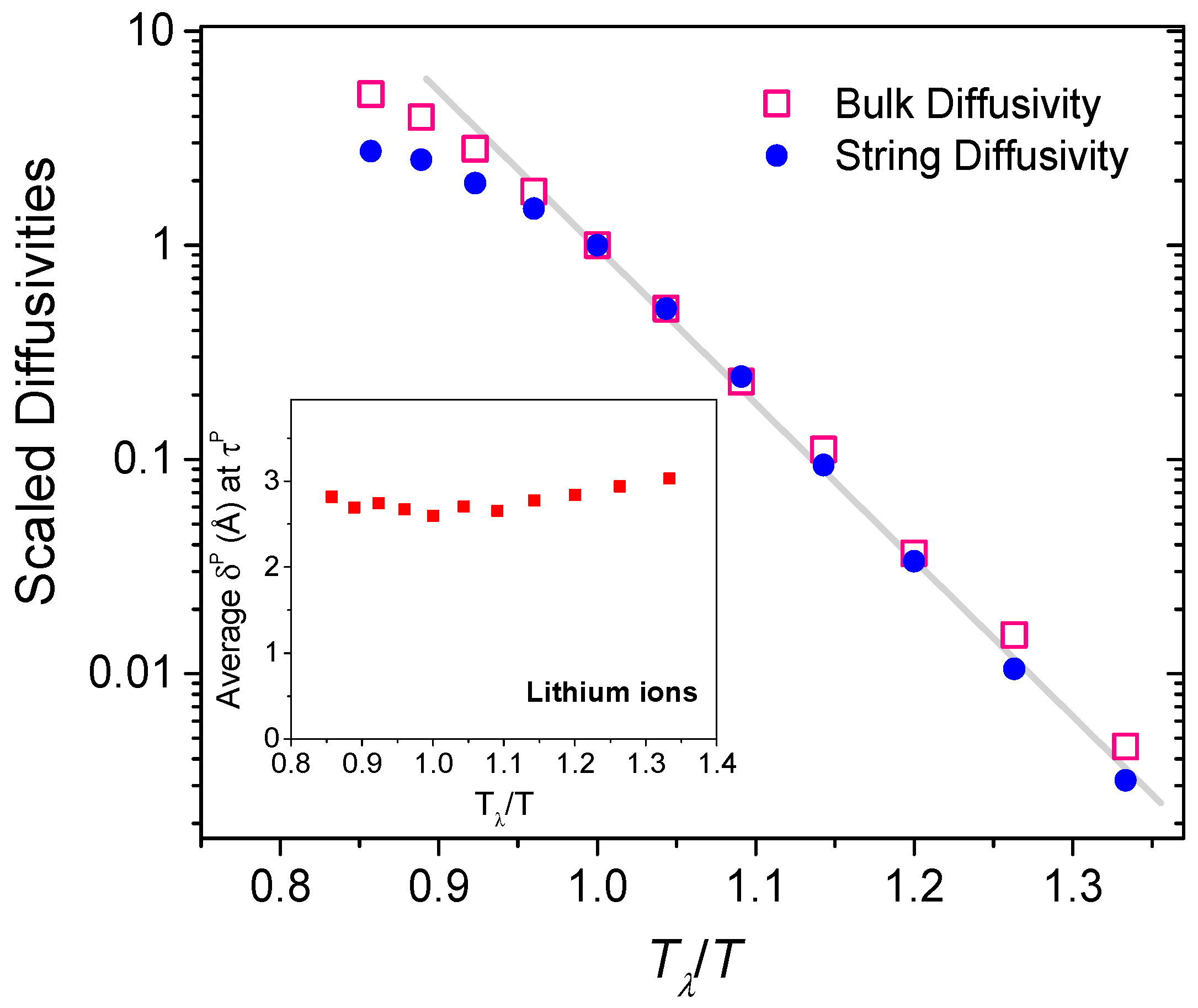
3.3. String-Like Dynamic Structures
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| z+ (|e|) | z− (|e|) | A++ (eV) | A−− (eV) | A+− (eV) | ρ+− (Å) |
|---|---|---|---|---|---|
| 0.790909 | −1.581818 | 0.0 | 0.0 | 1425.4833 | 0.236303 |
Appendix B. Comparison of Wolf and Ewald Summation Methods for Coulombic Interactions
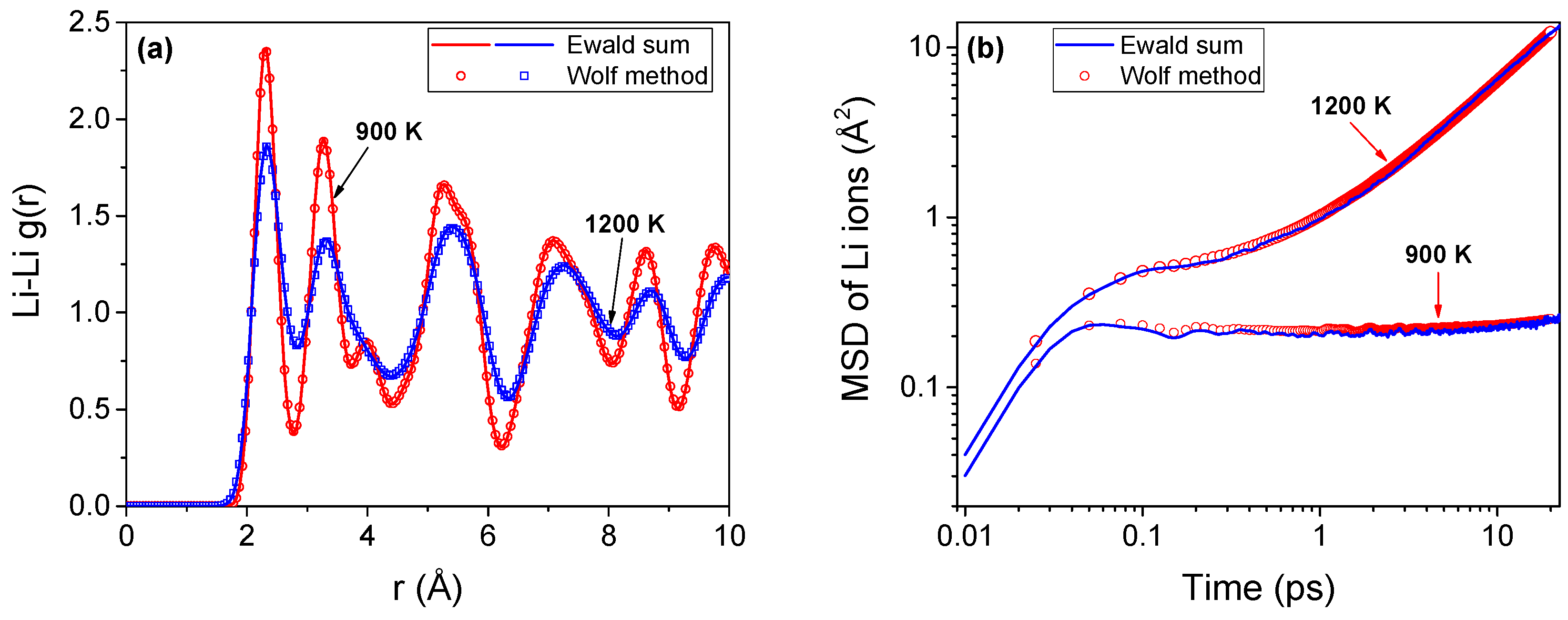
References
- Stacey, W.M. Fusion: An Introduction to the Physics and Technology of Magnetic Confinement Fusion, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Strange, J.H.; Rageb, S.M.; Chadwick, A.V.; Flack, K.W.; Harding, J.H. Conductivity and NMR study of ionic mobility in lithium oxide. Faraday Trans. 1990, 86, 1239–1241. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, J.; Leese, R.; Fellner, J.P.; Rodrigues, S.J.; Abraham, K.M. A solid-state, rechargeable, long cycle life lithium-air battery. J. Electrochem. Soc. 2010, 157, A50–A54. [Google Scholar] [CrossRef]
- Xu, M.; Ding, J.; Ma, E. One-dimensional stringlike cooperative migration of lithium ions in an ultrafast ionic conductor. Appl. Phys. Lett. 2012, 101, 031901. [Google Scholar] [CrossRef]
- Mo, Y.; Ong, S.P.; Ceder, G. First principles study of the Li10GeP2S12 lithium super ionic conductor material. Chem. Mater. 2012, 24, 15–17. [Google Scholar] [CrossRef]
- Salanne, M.; Marrocchelli, D.; Watson, G.W. Cooperative mechanism for the diffusion of Li+ ions in LiMgSO4F. J. Phys. Chem. C 2012, 116, 18618–18625. [Google Scholar] [CrossRef]
- Wang, Y.; Richards, W.D.; Ong, S.P.; Miara, L.J.; Kim, J.C.; Mo, Y.; Ceder, G. Design principles for solid-state lithium superionic conductors. Nat. Mater. 2015, 14, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.B.; Hassan, A.S.; Ramachandran, B.R.; Wick, C.D. Role of metal-lithium oxide interfaces in the extra lithium capacity of metal oxide lithium-ion battery anode materials. J. Electrochem. Soc. 2016, 163, A2172–A2178. [Google Scholar] [CrossRef]
- Hull, S. Superionics: Crystal structures and conduction processes. Rep. Prog. Phys. 2004, 67, 1233–1314. [Google Scholar] [CrossRef]
- Farley, T.W.D.; Hayes, W.; Hull, S.; Hutchings, M.T.; Vrtis, M. Investigation of thermally induced Li+ ion disorder in Li2O using neutron diffraction. J. Phys. Condens. Matter 1991, 3, 4761–4781. [Google Scholar] [CrossRef]
- Hutchings, M.T.; Clausen, K.; Dickens, M.H.; Hayes, W.; Kjems, J.K.; Schnabel, P.G.; Smith, C. Investigation of thermally induced anion disorder in fluorites using neutron scattering techniques. J. Phys. C Solid State Phys. 1984, 17, 3903–3940. [Google Scholar] [CrossRef]
- Voronin, B.M.; Volkov, S.V. Ionic conductivity of fluorite type crystals CaF2, SrF2, BaF2, and SrCl2 at high temperatures. J. Phys. Chem. Solids 2001, 62, 1349–1358. [Google Scholar] [CrossRef]
- Annamareddy, A.; Eapen, J. Disordering and dynamic self-organization in stoichiometric UO2 at high temperatures. J. Nucl. Mater. 2017, 483, 132–141. [Google Scholar] [CrossRef]
- Hull, S.; Norberg, S.T.; Ahmed, I.; Eriksson, S.G.; Mohn, C.E. High temperature crystal structures and superionic properties of SrCl2, SrBr2, BaCl2 and BaBr2. J. Solid State Chem. 2011, 184, 2925–2935. [Google Scholar] [CrossRef]
- Dworkin, A.S.; Bredig, M.A. Diffuse transition and melting in fluorite and anti-fluorite type of compounds: Heat content of potassium sulfide from 298 to 1260° K. J. Phys. Chem. 1968, 72, 1277–1281. [Google Scholar] [CrossRef]
- Eapen, J.; Annamareddy, A. Entropic crossovers in superionic fluorites from specific heat. Ionics 2017, 23, 1043–1047. [Google Scholar] [CrossRef]
- Catlow, C.R.A.; Comins, J.D.; Germano, F.A.; Harley, R.T.; Hayes, W. Brillouin scattering and theoretical studies of high-temperature disorder in fluorite crystals. J. Phys. C Solid State Phys. 1978, 11, 3197–3212. [Google Scholar] [CrossRef]
- Kleppmann, W.G. High-temperature disorder and the elastic constants in fluorite crystals. J. Phys. C Solid State Phys. 1978, 11, L91–L95. [Google Scholar] [CrossRef]
- Oishi, Y.; Kamei, Y.; Akiyama, M.; Yanagi, T. Self-diffusion coefficient of lithium in lithium oxide. J. Nucl. Mater. 1979, 87, 341–344. [Google Scholar] [CrossRef]
- Wilson, M.; Jahn, S.; Madden, P.A. The construction and application of a fully flexible computer simulation model for lithium oxide. J. Phys. Condens. Matter 2004, 16, S2795–S2810. [Google Scholar] [CrossRef]
- Annamareddy, V.A.; Nandi, P.K.; Mei, X.; Eapen, J. Waxing and waning of dynamical heterogeneity in the superionic state. Phys. Rev. E 2014, 89, 010301(R). [Google Scholar] [CrossRef] [PubMed]
- Annamareddy, A.; Eapen, J. Mobility propagation and dynamic facilitation in superionic conductors. J. Chem. Phys. 2015, 143, 194502. [Google Scholar] [CrossRef] [PubMed]
- Annamareddy, A.; Eapen, J. Low dimensional string-like relaxation underpins superionic conduction in fluorites and related structures. Sci. Rep. 2017, 7, 44149. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Freeman, C.M.; Saxe, P.; Wimmer, E. Thermal expansion, diffusion and melting of Li2O using a compact forcefield derived from ab initio molecular dynamics. Model. Simul. Mater. Sci. Eng. 2014, 22, 075009. [Google Scholar] [CrossRef]
- Goel, P.; Choudhury, N.; Chaplot, S.L. Fast ion diffusion, superionic conductivity and phase transitions of the nuclear materials UO2 and Li2O. J. Phys. Condens. Matter 2007, 19, 386239. [Google Scholar] [CrossRef]
- Goel, P.; Choudhury, N.; Chaplot, S.L. Superionic behavior of lithium oxide Li2O: A lattice dynamics and molecular dynamics study. Phys. Rev. B 2004, 70, 174307. [Google Scholar] [CrossRef]
- Mulliner, A.D.; Aeberhard, P.C.; Battle, P.D.; David, W.I.F.; Refson, K. Diffusion in Li2O studied by non-equilibrium molecular dynamics for 873 < T/K < 1603. Phys. Chem. Chem. Phys. 2015, 17, 21470–21475. [Google Scholar] [PubMed]
- Rodeja, J.G.; Meyer, M.; Hayoun, M. Derivation and validation of model potentials for Li2O from density-functional theory. Model. Simul. Mater. Sci. Eng. 2001, 9, 81–96. [Google Scholar] [CrossRef]
- Oda, T.; Oya, Y.; Tanaka, S.; Weber, W.J. Validation of potential models for Li2O in classical molecular dynamics simulation. J. Nucl. Mater. 2007, 367, 263–268. [Google Scholar] [CrossRef]
- Wolf, D.; Keblinski, P.; Phillpot, S.R.; Eggebrecht, J. Exact method for the simulation of Coulombic systems by spherically truncated, pairwise r−1 summation. J. Chem. Phys. 1999, 110, 8254–8282. [Google Scholar] [CrossRef]
- Rapaport, D.C. The Art of Molecular Dynamics Simulation, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Hochrein, O.; Zahn, D. Atomic mechanisms of superionic conductivity in fluorite. Solid State Ionics 2009, 180, 116–119. [Google Scholar] [CrossRef]
- Lunev, A.V.; Tarasov, B.A. A classical molecular dynamics study of the correlation between the Bredig transition and thermal conductivity of stoichiometric uranium dioxide. J. Nucl. Mater. 2011, 415, 217–221. [Google Scholar] [CrossRef]
- Gebremichael, Y.; Vogel, M.; Glotzer, S.C. Particle dynamics and the development of string-like motion in a simulated monoatomic supercooled liquid. J. Chem. Phys. 2004, 120, 4415–4427. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.; Douglas, J.F.; Kob, W.; Plimpton, S.J.; Poole, P.H.; Glotzer, S.C. Stringlike cooperative motion in a supercooled liquid. Phys. Rev. Lett. 1998, 80, 2338. [Google Scholar] [CrossRef]
- Keys, A.S.; Abate, A.R.; Glotzer, S.C.; Durian, D.J. Measurement of growing dynamical length scales and prediction of the jamming transition in a granular material. Nat. Phys. 2007, 3, 260–264. [Google Scholar] [CrossRef]
- Clausen, K.; Hayes, W.; Macdonald, J.E.; Osborn, R.; Hutchings, M.T. Observation of oxygen Frenkel disorder in uranium dioxide above 2000 K by use of neutron-scattering techniques. Phys. Rev. Lett. 1984, 52, 1238–1241. [Google Scholar] [CrossRef]
- Allnatt, A.R.; Chadwick, A.V.; Jacobs, P.W.M. A model for the onset of fast-ion conduction in fluorites. Proc. R. Soc. Lond. Ser. A 1987, 410, 385–408. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19, (Version 9 December 2014). [Google Scholar] [CrossRef]
| Experiments | Atomistic (MD) Simulations | |
|---|---|---|
| Tα (K) | 1000 [2,10] | 1000 † |
| Tλ (K) | 1200 [10] | 1200 [24] |
| Tm (K) | 1711 [24] | 1715 [24] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annamareddy, A.; Eapen, J. Ion Hopping and Constrained Li Diffusion Pathways in the Superionic State of Antifluorite Li2O. Entropy 2017, 19, 227. https://doi.org/10.3390/e19050227
Annamareddy A, Eapen J. Ion Hopping and Constrained Li Diffusion Pathways in the Superionic State of Antifluorite Li2O. Entropy. 2017; 19(5):227. https://doi.org/10.3390/e19050227
Chicago/Turabian StyleAnnamareddy, Ajay, and Jacob Eapen. 2017. "Ion Hopping and Constrained Li Diffusion Pathways in the Superionic State of Antifluorite Li2O" Entropy 19, no. 5: 227. https://doi.org/10.3390/e19050227





