2. Thermodynamics, Replica Energy, Statistical Ensembles
To obtain the thermodynamic properties of a system from the principles of statistical mechanics, one considers a great number
of independent replicas of the system, namely, a statistical ensemble. The replicas are identical in nature, but they differ in phase, that is, in their condition with respect to configuration and velocity [
20]. If the energy, entropy, volume and number of particles of the system under consideration are
E,
S,
V and
N, respectively, the corresponding quantities of the ensemble are
,
,
and
. Energy variations in the ensemble satisfy the general thermodynamic relation [
3]
where
T is the temperature,
P is the pressure exerted on the boundary of the systems, and
is the chemical potential of a single system. The last term on the right-hand side of Equation (
1) accounts for the energy variation when the number of members of the ensemble
varies, holding
,
and
constant. The replica energy
, formally given by
vanishes if the system is additive; this can be derived using the fact that for additive systems the extensive variables are linear homogeneous functions of the system size [
19]. The physical reason why the replica energy
does not vanish for nonadditive systems can be traced back to the following argument. By changing the size of a macroscopic nonadditive system, its bulk properties in general change, while this does not occur, as it is well known, for an additive system (that is why the extensive variables are linear homogeneous functions of the systems size for additive systems). The replica energy is a measure of the energy associated with this change and Equation (
2) is the mathematical expression of this fact.
In the case in which all single systems properties are held constant, Equation (
1) can be written
, which can be integrated from 0 to
to give
. Dividing by
the latter equation gives
which relates the properties of a single system with the replica energy. By differentiation, one obtains:
Thus, one can exploit the first law of thermodynamics, expressed by
to obtain
The above equation generalizes the usual Gibbs–Duhem equation for additive systems, which is obtained by setting to zero the left-hand side of Equation (
6). Since, in general, the usual Gibbs–Duhem equation does not hold for nonadditive systems [
21], there exists the possibility of taking
T,
P and
as independent variables, a fact that is forbidden when
. Moreover, as can be seen from Equation (
3), we highlight that when
, the Gibbs free energy
is not equal to
.
Depending on the control parameters defining the state of the system, certain quantities fluctuate and other quantities are fixed. Distinguishing between these two kinds of quantities is relevant here, and it is convenient to set now the notation that will be used to indicate such a distinction when necessary: if the energy
E, volume
V, or number of particles
N are not control parameters, they are fluctuating quantities and will be denoted with a bar by
,
, and
, respectively. Equations (
3), (
5), and (
6) are general relations at a thermodynamic level and have to be understood for quantities with or without bars.
We will refer to the variables E, V, and N as constraint variables, and ensembles in which at least one of the constraint variables is a control parameter will be termed as constrained ensembles. The thermodynamic properties of an isolated system are obtained from a completely constrained ensemble in which all the constraint variables are control parameters; as is well known, this is the microcanonical ensemble. On the other hand, if none of the constraint variables is a control parameter, the system is said to be completely open and the associated ensemble is the unconstrained ensemble.
We have reminded above that ensemble inequivalence is associated with the fact that, for nonadditive systems, the possible equilibrium configurations depend on the specific control parameters used to define its state. Therefore, the thermodynamics of the system must be necessarily derived from the characteristic function (the entropy or the free energies) in the ensemble associated with the particular set of control parameters under consideration. In doing so, it is in general possible to obtain the replica energy from the corresponding characteristic function, except in the case where the replica energy itself is the characteristic function corresponding to a particular set of control parameters. In fact, the replica energy is the free energy associated with the unconstrained ensemble where the corresponding control parameters are
T,
P and
[
22], namely,
where
is the unconstrained partition function,
being the microcanonical density of states, which is defined below, and
the Boltzmann constant. In
Section 4, we will come back to the relation between the microcanonical density of states (and the associated microcanonical entropy) and the replica energy.
The replica energy can indeed be explicitly computed in a few physical examples. For instance, given the potential energy
where
is the particle density and
is the two-body potential of a system embedded in a
d-dimensional space, the replica energy can be evaluated to be [
23]
where
is the excess pressure due to the interactions. In the particular case in which
is a long-range potential decaying as
with
, one has [
21]
, showing that the replica energy vanishes when
, which corresponds to the limit of an additive system.
Another remarkable example is the extended Thirring model [
22], representing the physics of self-gravitating systems, for which the potential energy is
where
is the number of particle inside a given volume
and
are those outside (
and
b are parameters of the model). The expression of the replica energy
is given by Equation (
10) and can be written as
3. Response Functions and Ensemble Inequivalence
In this and in the next section, we analyze the relation between ensemble inequivalence and the occurrence of negative response functions. Ensemble inequivalence can be studied with the help of the properties of the Legendre–Fenchel transformation; this approach, already well documented for constrained ensembles [
24,
25,
26], can be extended to the case of the unconstrained ensemble. It is the Legendre–Fenchel transformation that allows one to connect ensemble inequivalence and negative response functions. In this paper, we are particularly interested in the inequivalence between the unconstrained ensemble and the other ensembles. However, it is instructive to consider first the inequivalence between constrained ensembles; this will be done in this section, showing the associated anomalous response functions. The unconstrained ensemble will be considered in
Section 4. In the following, we use units in which the Boltzmann constant
is set to unity.
To begin, we consider the microcanonical and canonical ensembles for a system described by the Hamiltonian
with
and
, where
and
are the momentum and position of particle
i, respectively, and
d is the dimensionality of the system. Just for completeness, we remind readers that, while the constraint variables
E,
V and
N are the control parameters of the microcanonical ensemble, the control parameters of the canonical ensemble are
T,
V and
N. The microcanonical density of states is given by
where
h is a constant and
, while the canonical partition function is
Taking advantage of the Dirac
in (
13), after posing
, we rewrite the canonical partition function as
with
being the inverse canonical temperature,
the rescaled Helmholtz free energy, and
, the logarithm of the density of states
, the microcanonical entropy. In the large
N limit, we can compute the integral on the right-hand side of Equation (
15) using the saddle-point approximation and write
We thus obtain the rescaled Helmholtz free energy as the Legendre–Fenchel transform of the microcanonical entropy with respect to the energy [
1,
2,
24,
25,
26], which reduces to the usual Legendre transformation if the entropy is differentiable and concave in
E at constant
V and
N.
On the one hand, the Legendre–Fenchel transformation of any function, as defined in (
16), is always a globally concave function [
26]. This very remarkable property guarantees that the rescaled free energy
is always globally concave with respect to
. For convenience, we recall in
Appendix A the definition of locally and globally concave (and convex) functions, of concave (and convex) envelope, together with some properties of the Legendre–Fenchel transformation and its relations with concave functions. In the following, we will refer to these definitions and relations several times, therefore the reader not familiar with them should read
Appendix A at this point. Using that
the concavity of
with respect to
means that
which ensures that the response function
that is, the heat capacity, is a nonnegative quantity in the canonical ensemble. This statement is valid regardless of the differentiability of
. If
is twice-differentiable, then
is continuous; otherwise, it has discontinuities, or it can even diverge, if
is not differentiable, for the values of
where the derivative of this function is not continuous; however, the inequality in Equation (
19) is always satisfied in the canonical ensemble, since
is always globally concave. For additive systems, the heat capacity is a nonnegative quantity also in the microcanonical ensemble (furthermore, it coincides with that in the canonical ensemble), since for these systems it can be proved that the microcanonical entropy
is globally concave with respect to
E [
27] and, as remarked in
Appendix A, globally concave functions coincide with their concave envelope. Actually, it can be proved that, for additive systems,
is globally concave also with respect to
V, and, in addition, it is globally completely concave in the
plane [
27]. On the other hand, the lack of additivity can induce the lack of global concavity in the microcanonical entropy as a function of the energy. Hence, the quantity
can be negative in the microcanonical ensemble (hereafter, we use, for simplicity, the same symbol to represent the response functions in the different ensembles). Referring to
Figure A1 in
Appendix A, we have a negative microcanonical heat capacity
for a range of
E values if the entropy has a behavior similar to that of the upper curve or the middle curve; if the behavior is similar to that of the lower curve, the heat capacity is positive except for the
E value where the cusp occurs, and where it is not defined. Note that the middle curve presents both features shown separately by the other two curves: it has a range of
E where
is negative and also a point of discontinuity. In all these cases, the microcanonical entropy does not coincide with its concave envelope; its Legendre–Fenchel transform, i.e., the function
, will have at least a point
, for the given
V and
N values, where its first derivative with respect to
is not defined (see
Appendix A). Thus, also the associated response function, the heat capacity, is not defined there. This point marks the occurrence of a first order phase transition in the canonical ensemble. We remark that, apart from such points, the canonical heat capacity (
19) is perfectly defined and always positive. Negative heat capacities in the microcanonical ensemble can occur since they are not forbidden by any fundamental requirement. In addition, according to equation (
19), equilibrium states with negative heat capacity cannot be realized if the system is put in contact with an infinite thermal bath (canonical ensemble). It is therefore clear that states associated with energy values where the entropy does not coincide with its concave envelope have no correspondence in the canonical ensemble.
To summarize the main result, if the microcanonical entropy does not coincide with its concave envelope with respect to
E, the microcanonical and canonical ensembles are not equivalent [
1,
2,
24,
25,
26,
28]. In this case, the function
presents at least a point of discontinuous derivative with respect to
, associated with a first order phase transition. The function
has a discontinuous derivative with respect to
also in the limiting case where the microcanonical entropy does coincide with its concave envelope, but the latter is a linear function of the energy in a given interval. This case has been referred to as “partial equivalence” [
29] because there is equivalence but not one-to-one: a single value of
corresponds to a whole interval of values of the energy. This may happen also in additive systems and indeed it happens whenever the system undergoes a discontinuous phase transition, e.g., when there is a change of state like a liquid–gas phase transition.
It is useful to stress the physical reason that permits having a negative heat capacity in the microcanonical ensemble, while this is not allowed in the canonical ensemble. In the microcanonical ensemble, the energy is fixed, and it can be given a value belonging to the energy range of convexity. In the canonical ensemble, the energy can fluctuate, and it can be easily seen that, if a system at a given energy
E in the energy range of convexity, with expected value of the temperature equal to
, is put in contact with a heat bath at temperature
, it is unstable with respect to energy fluctuations, and it will acquire an expected value of the energy where the associated temperature is also
, but that it is located in an energy range of concavity of the microcanonical entropy. A state with an energy where the microcanonical entropy is locally concave, but that does not belong to the range where it coincides with its concave envelope, is metastable when put in contact with a heat bath at the corresponding temperature
, i.e., it is stable with respect to sufficiently small energy fluctuations, but not with respect to general fluctuations; namely, it is not globally stable and then it cannot be defined as an equilibrium state [
1].
In the microcanonical ensemble, nonadditive systems could exhibit a convex region in the entropy as a function of the other constraint variables,
V or
N, or, more generally, ranges where the entropy does not coincide with its concave envelope with respect to one or both of these variables (while for additive systems the entropy is globally concave with respect to
V and
N). For those variables, such anomalous behavior is inherited by the canonical ensemble, since in this case both
V and
N are control parameters as well, and the Legendre–Fenchel transformation does not involve them. However, we point out the following. While “normal” behavior of the microcanonical entropy
is represented by global concavity with respect to
V and
N, “normal” behavior of the rescaled Helmholtz free energy
(or of the free energy
) is represented by global convexity with respect to these variables, since, in the Legendre–Fenchel transform (
16), the microcanonical entropy appears with the minus sign. Thus, in nonadditive systems where the microcanonical entropy can have ranges of convexity in
V and/or in
N, correspondingly, the Helmholtz free energy will have ranges of concavity in
V and/or in
N.
Let us now turn to the grand canonical ensemble. In this ensemble, in addition to the energy, the number of particles is also not constrained. The control parameters of this ensemble are
,
T and
V. The grand canonical partition function
can be written as
where
. The rescaled grand potential
is thus given by the term that dominates the sum according to
which is the Legendre–Fenchel transform of
with respect to
N. This expression (
22) assures that
is always globally concave in
, and that its concavity with respect to
is inherited from that of
. Using Equation (
16), we can also write
From this expression, we infer that, in addition,
is globally completely concave in the plane
. Thus, from
we have
so that in the grand canonical ensemble
Here,
is a response function, just as the heat capacity; it tells us that, in the grand canonical ensemble, the number of particles increases whenever the chemical potential increases, holding
T and
V constant. We can repeat here the observation made for the canonical heat capacity (
19). Thus, the positivity of
in the grand canonical ensemble is valid regardless of the differentiability of
. If
is twice-differentiable, then
is continuous; otherwise, it has discontinuities, or it can even diverge, if
is not differentiable, for the values of
where the derivative of this function is not continuous; however, the inequality in Equation (
26) is always satisfied in the grand canonical ensemble since
is always globally concave.
As a side remark, we note that this response function can be written as
, where
is the thermodynamic factor given by [
30]
In addition, for macroscopic short-range interacting systems, the usual Gibbs–Duhem equation holds, and the function
can be directly related to the isothermal compressibility
. For this kind of systems, using
, we can write
Since the Gibbs–Duhem holds in this case (
), under isothermal conditions, we have
. Hence, using the fact that
,
is related to
according to
so that the sign of
and that of
are the same, namely, they are both positive quantities. However, if the replica energy is different from zero, as in nonadditive systems, the signs of these response functions are independent from each other, in general.
Concerning the issue of ensemble inequivalence, in the canonical ensemble, there is no mechanism ensuring that for nonadditive systems the Helmholtz free energy is convex with respect to
N. Therefore, the quantity
could be negative. Again, in perfect analogy to the relation between microcanonical and canonical ensembles, we have the following: if the rescaled Helmholtz free energy does not coincide with its convex envelope with respect to
N, then its convex Legendre–Fenchel transform
will have at least a point
, for the given
V and
values, where its first derivative with respect to
is not defined, marking the occurrence of a first order phase transition. Apart from this isolated point, or points,
is perfectly defined and always positive in the grand canonical ensemble. On the other hand, the response function
in the canonical ensemble can be negative, if the rescaled Helmholtz free energy has a range where it is not locally convex with respect to
N, or could have points where it is not defined, or both; these three cases correspond to the upper, lower and middle curves in
Figure A1, respectively.
Summarizing the main result, if the rescaled Helmholtz free energy does not coincide with its convex envelope with respect to N, the canonical and grand canonical ensembles are not equivalent. In this case, the function presents at least a point of discontinuous derivative with respect to , associated with a first order phase transition. If an equilibrium canonical state in which the rescaled Helmholtz free energy does not coincide with its convex envelope with respect to N is put in contact with a reservoir with its same chemical potential and with which it can exchange particles, then it becomes either unstable or not globally stable.
We now consider the isothermal-isobaric ensemble, where the volume is not a control parameter; the control parameters are
N,
T and
P. The associated partition function
is written as
where
. Hence, the saddle-point approximation gives the rescaled Gibbs free energy
as
which is the Legendre–Fenchel transform of
with respect to
V. Moreover, using (
16), we can also write
from which we infer that
is concave in both
and
; moreover, it is completely concave in the plane
. In particular, using that
we can assert that
and therefore that the isothermal compressibility in the isothermal-isobaric ensemble is nonnegative,
This is what we expect on physical grounds, since states with negative
cannot be stable under volume fluctuations. For convenience, instead of the isothermal compressibility
, we can consider the quantity
as a response function, where the subscript
N is written to emphasize that it is also computed at constant number of particles. Then, in the isothermal-isobaric ensemble
The same argument made before applies. Thus, the positivity of
in the isothermal-isobaric ensemble is valid regardless of the differentiability of
. If
is twice-differentiable, then
is continuous, otherwise it has discontinuities, or it can even diverge, if
is not differentiable, for the values of
P where the derivative of this function is not continuous; however, the inequality in Equation (
35) is always satisfied in the isothermal-isobaric ensemble, since
is always globally concave. However, in the canonical ensemble, the volume is a control parameter, i.e., it is fixed in the equilibrium configuration. The Helmholtz free energy for nonadditive systems is not necessarily convex with respect to
V, so that states with negative isothermal compressibility or, equivalently, negative
can be realized. In fact, in the canonical ensemble, we have
which is not restricted to be a positive quantity. An argument analogous to that already used before implies the following. If the rescaled Helmholtz free energy does not coincide with its convex envelope with respect to
V, then its convex Legendre–Fenchel transform
will have at least a point
, for the given
N and
values, where its first derivative with respect to
is not defined, marking the occurrence of a first order phase transition. Apart from this isolated point, or points,
is perfectly defined and always positive in the isothermal-isobaric ensemble. On the other hand, the response function
in the canonical ensemble can be negative at points where the rescaled Helmholtz free energy is not locally convex with respect to
V, or could have points where it is not defined, or both (the three cases represented in
Figure A1).
According to the previous discussion, we conclude that, if the rescaled Helmholtz free energy does not coincide with its convex envelope with respect to V, the canonical and isothermal-isobaric ensembles are not equivalent. In this case, the function presents at least a point of discontinuous derivative with respect to , associated with a first order phase transition. If an equilibrium canonical state in which the rescaled Helmholtz free energy does not coincide with its convex envelope with respect to V is put in contact with an environment with its same pressure, then it becomes either unstable or not globally stable.
4. From Microcanonical Entropy to Rescaled Replica Energy
Continuing the discussion of the preceding section, here we focus on the unconstrained ensemble and its connection with the other ensembles. Since the unconstrained ensemble describes the thermodynamics of completely open systems, it can be seen as the opposite situation of the one described by the microcanonical ensemble where the systems are isolated. Such an opposite situation is reflected in the curvature properties of the thermodynamic characteristic functions. We shall see that the characteristic function of completely open systems, the rescaled replica energy, possesses always a very well defined concavity with respect to all its natural variables (none of them being a constraint variable), while, as noted previously, the microcanonical entropy can be nonconcave in any of its natural variables (all of them being constraint variables).
In the case where the energy, volume, and number of particles fluctuate, from (
8), we can write the unconstrained partition function
as a function of the microcanonical entropy, that is,
Similarly to the other ensembles, the rescaled replica energy
and the replica energy
are related by
. We note that considering the set of control parameters
,
, and
is completely equivalent to considering
T,
P, and
. Evaluating (
37) in a saddle-point approximation, we have
which ensures that
is completely concave, implying that it is also separately concave in
,
, and
. Therefore, in the unconstrained ensemble, we get
Using that
,
, and
, Equations (
39), (
40), and (
41) imply that the response functions
respectively, are nonnegative in the unconstrained ensemble. As before, there could be isolated points where these response functions are not defined; apart from these isolated points, the response functions are perfectly defined and are always positive.
The rescaled replica energy can be related to the rescaled grand potential via
Using the saddle-point approximation, we then have
so that
is expressed as the Legendre–Fenchel transform of
with respect to
V. The grand potential is not necessarily a convex function in
V for nonadditive systems, so that the response function
, given in the grand canonical ensemble by
can be a negative quantity. If the grand potential does not coincide with its convex envelope with respect to
V, the grand canonical and unconstrained ensembles are not equivalent. As before, the response function
in the grand canonical ensemble can be negative where the rescaled grand potential is not locally convex with respect to
V, or could have points where it is not defined, or both (the three cases of
Figure A1).
We observe here that, if the rescaled grand potential energy does not coincide with its convex envelope with respect to V, the grand canonical and the unconstrained ensembles are not equivalent. In this case, the function presents at least a point of discontinuous derivative with respect to , associated with a first order phase transition. If an equilibrium grand canonical state in which the rescaled grand potential does not coincide with its convex envelope with respect to V is put in contact with an environment with its same pressure, then it becomes either unstable or not globally stable.
Furthermore, we can also write
which relates the rescaled replica energy to the rescaled Gibbs free energy, and therefore we obtain
as the Legendre–Fenchel transform of
with respect to
N,
Now, let us consider the response function
in the isothermal-isobaric ensemble. The rescaled Gibbs free energy is not necessarily convex in
N for nonadditive systems, so that
is not restricted to be a positive quantity. The unconstrained and isothermal-isobaric ensembles are not equivalent if the Gibbs free energy does not coincide with its convex envelope with respect to
N. The response function
in the isothermal-isobaric ensemble can be negative at points where the rescaled Gibbs free energy is not locally convex with respect to
N, or could have points where it is not defined, or both (the three cases of
Figure A1).
Analogously with the previous situations, we point out here that, if the rescaled Gibbs free energy energy does not coincide with its convex envelope with respect to N, the isothermal-isobaric and the unconstrained ensembles are not equivalent. In this case, the function presents at least a point of discontinuous derivative with respect to , associated with a first order phase transition. If an equilibrium isothermal-isobaric state in which the rescaled Gibbs free energy does not coincide with its convex envelope with respect to N is put in contact with an environment with its same chemical potential and with which it can exchange particles, then it becomes either unstable or not globally stable.
A final remark. We have noted that the replica energy vanishes for additive systems. This is related to the fact that the validity of the Gibbs–Duhem equation implies that the variables cannot be taken as independent control parameters for those systems. In turn, this implies that is negligible in the thermodynamic limit.
5. Discussion
We have seen that ensemble inequivalence is connected with the occurrence of negative response functions, and that these anomalous responses are in turn associated with anomalous concavity properties of the thermodynamic functions. In detail, we note that all these response functions concern the variation of a constraint variable (
E,
V or
N) with respect to the respective conjugate thermodynamic variable (
T,
P and
, respectively). In addition, the Legendre–Fenchel transformations relating the various thermodynamic functions are defined by the minimization with respect one of the constraint variables. In
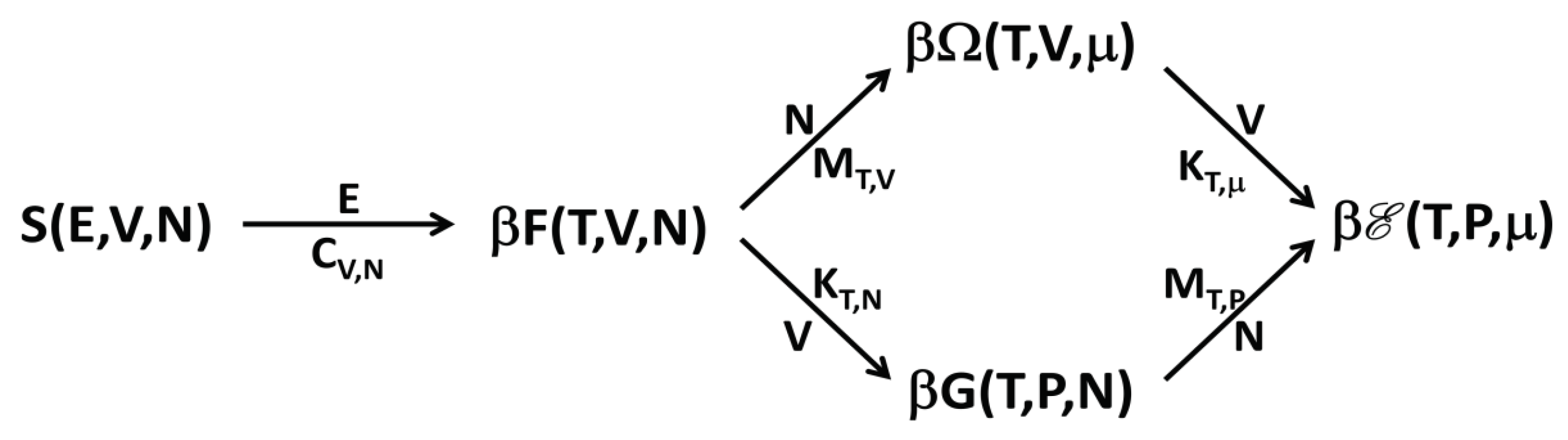
Figure 1, we show a simple scheme of the transformations and of the response functions connecting the different thermodynamic potentials.
It is interesting to note the following. The rescaled replica energy
is obtained from the microcanonical entropy by minimizing with respect to all the constraint variables. However, while the first minimization with respect to
E produces the rescaled Helmholtz free energy
, the following minimizations with respect to
N and
V can be made in the two different orders, thus producing, as “intermediate” thermodynamic functions, either
or
. For this reason, the scheme in
Figure 1 has two routes from
S to
.
We stress once more that a negative response function implies ensemble inequivalence, while the reverse is not true: ensemble inequivalence can occur with or without a negative response function. We have described in each case which response function can be negative, and, referring to
Figure A1, we have cited the possible situations, clarifying that ensemble inequivalence implies the presence of a first order phase transition in the ensemble which is the arriving one in the Legendre–Fenchel transformation. We also note that, very often, concrete models can present all the three cases considered in
Figure A1, which occur varying the value of the parameters of the Hamiltonian. Thus, in conclusion: ensemble inequivalence occurs each time a thermodynamic function does not coincide with its concave or, alternatively (depending on the concrete function, see the analyses above), convex envelope with respect to a constraint variable (also the constraint variable of interest depends on the concrete function, see
Figure 1); a negative response function can be present in these cases, but it cannot occur with ensemble equivalence.
In principle, one may wonder about the following point. Is it possible that an ensemble corresponding to a thermodynamic function that is the starting one in a Legendre–Fenchel transformation is not equivalent to the ensemble corresponding to the arriving function, but, at the same time, it is equivalent to the ensemble corresponding to a successive function of the scheme? For more clarity and, as an example, referring to
Figure 1: is it possible that the microcanonical ensemble, corresponding to
S, is not equivalent to the canonical ensemble, corresponding to
, but it is equivalent either to the grand canonical ensemble, corresponding to
, or the isothermal-isobaric ensemble, corresponding to
? This situation is not possible and it can be seen in the following way. Suppose that the microcanonical ensemble is equivalent to the grand canonical ensemble. This means that Equation (
23) can be inverted, obtaining
i.e., the microcanonical entropy
coincides with its concave envelope with respect to the double Legendre–Fenchel transformation in the
plane. This implies that
is globally concave in the
plane, but then it is a fortiori globally concave with respect to
E, and it coincides with its concave envelope with respect to
E. In turn, this implies that the microcanonical and the canonical ensembles are equivalent. Then, if the microcanonical ensemble is not equivalent to the canonical ensemble, it is not equivalent also to the grand canonical ensemble.
The same procedure can be used if one assumes that the microcanonical ensemble is equivalent to the isothermal-isobaric ensemble. Then, Equation (
31) can be inverted to have
i.e., the microcanonical entropy coincides with its concave envelope with respect to the double Legendre–Fenchel transformation in the
plane. This implies that
is globally concave in the
plane; however, then, as before, it is a fortiori globally concave with respect to
E and the microcanonical and canonical ensembles are equivalent. Then, if the microcanonical ensemble is not equivalent to the canonical ensemble, it is not equivalent also to the isothermal-isobaric ensemble.
The above derivations are valid regardless of the differentiability of the thermodynamic functions. It is instructive to also give an alternative derivation based on partial derivatives, which shows that the heat capacity at constant
V and
N is positive in both the grand canonical and isothermal-isobaric ensemble. This is not completely trivial, since
are not the control parameters of either of these two ensembles. In
Appendix B, we present this derivation.
In an analogous way, if the canonical ensemble is not equivalent to, e.g., the grand canonical ensemble, then it is not equivalent also to the unconstrained ensemble. On the contrary, it may happen that canonical and grand canonical ensembles are equivalent, but they are both not equivalent to the unconstrained ensemble (see Reference [
22] for a concrete example).
In this paper, we have presented a general discussion, without reference to any specific model. Although the results are valid regardless of the differentiability of the thermodynamic functions, as a matter of fact, the most interesting situations arise when we have points where the differentiability does not hold, i.e., when we are dealing with first-order phase transitions. In fact, if neither of the two ensembles connected by a Legendre–Fenchel transformation has a first order phase transition, but at most a continuous transition, then the two ensembles are equivalent.
The results here discussed have the consequence that with ensemble inequivalence the phase transitions are located, generally, in different points of the thermodynamic phase diagram for nonadditive systems. From the general results, one can also prove that, in many cases, it is possible to obtain the response function in the “higher” ensemble from that in the “lower” ensemble (where “higher” means that, in the scheme of
Figure 1, it is on the right of the “smaller” and connected by one or more arrows) by invoking the Maxwell construction. For example, if one computes the function
(at constant
V and
N) in the microcanonical ensemble and then obtains the specific heat
, then the analogous curve and the specific heat in the canonical ensemble are obtained by applying the Maxwell construction in the neighborhood of the regions where the microcanonical
is negative.
In any case, we believe that a general and simple scheme like the one given in this paper can be useful as a reference material when dealing with concrete nonadditive systems.