Influence of Chronic Obstructive Pulmonary Disease and Moderate-To-Severe Sleep Apnoea in Overnight Cardiac Autonomic Modulation: Time, Frequency and Non-Linear Analyses
Abstract
:1. Introduction
2. Material and Methods
2.1. Population under Study
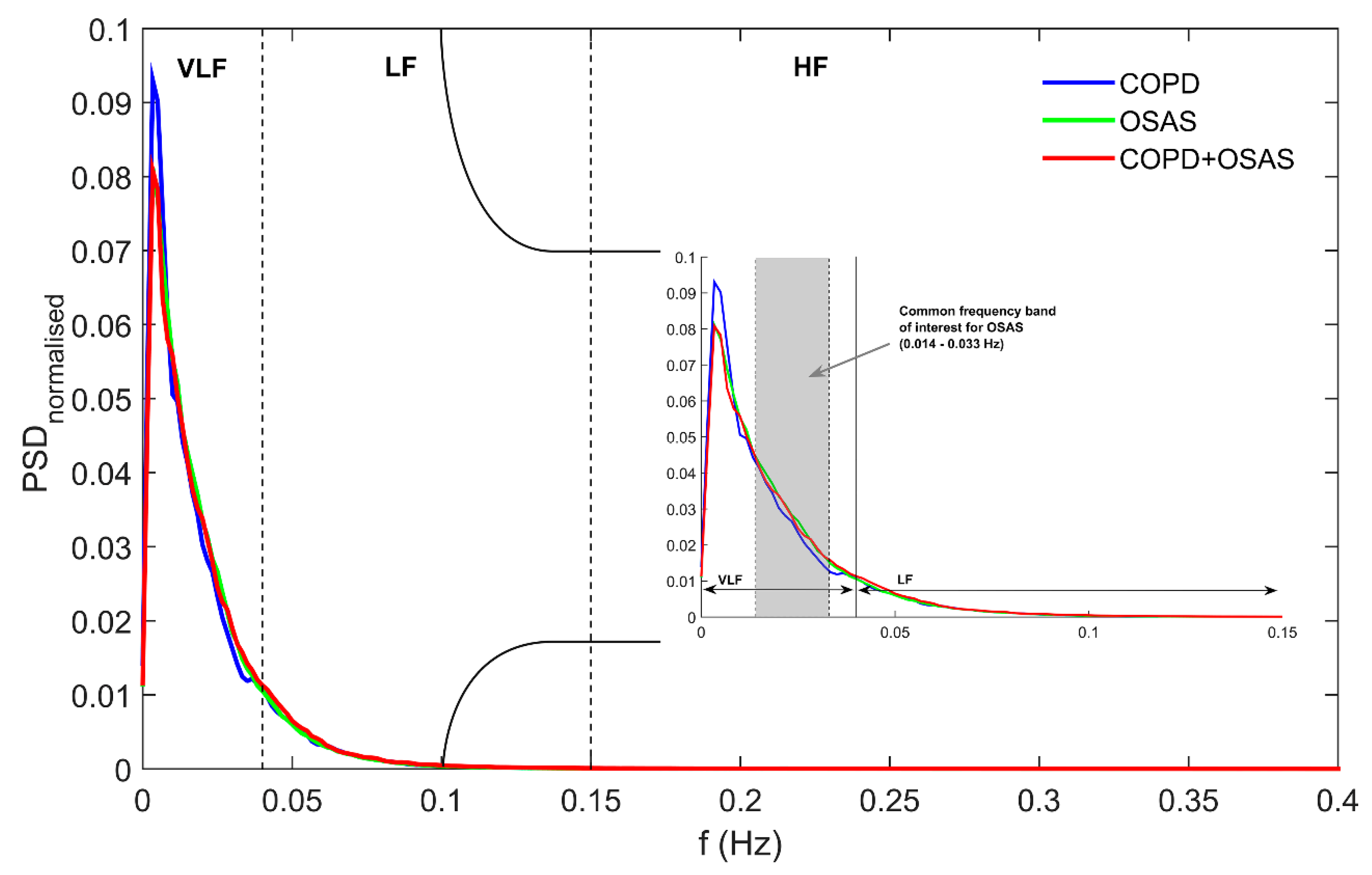
2.2. PRV Analysis
- -
- Average of pulse-to-pulse interval (AVNN). It is a global measure of the inter-beat interval (inverse of pulse rate).
- -
- Standard deviation of pulse-to-pulse interval (SDNN). It provides a global measure of variability.
- -
- Root mean square of successive differences of pulse-to-pulse intervals (RMSSD). It reflects vagal activity.
- -
- Very low frequency power (VLF). It measures rhythms with periodicities between 25 s and 5 min (0.0033–0.04 Hz). VLF reflects vagal and renin-angiotensin system effects on PR. It is usually normalised to the total power of the signal (VLFn).
- -
- Low frequency power (LF). It measures rhythms from 2.5 to 9 cycles per minute (0.04–0.15 Hz). LF is associated with both sympathetic and parasympathetic activity of the nervous system. It is commonly computed as the ratio to the cumulative spectral power in both the LF and HF bands (LFn).
- -
- High frequency power (HF). It captures rhythms with periodicities between 9 and 24 cycles per minute (0.15–0.40 Hz). HF is exclusively modulated by the parasympathetic nervous system reflecting vagal activity. As LF, it is usually measured as the ratio to the spectral power in the LF+HF band (HFn).
- -
- Low frequency to high frequency ratio (LF/HF). It measures the sympathetic component of PRV modulation reflecting the so-called sympathovagal balance.
- -
- Spectral power in the OSAS-related frequency band (OSASF). It reflects changes with periodicities between 30 s and 70 s (0.014–0.033 Hz). The power spectrum in this frequency band has been found to be related to the repetition of apnoeic events during the night [26,28]. It is usually normalised to the total signal power (OSASFn).
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2017 Report. Respirology 2017, 22, 575–601. [Google Scholar] [CrossRef] [Green Version]
- Halbert, R.J.; Natoli, J.L.; Gano, A.; Badamgarav, E.; Buist, A.S.; Mannino, D.M. Global burden of COPD: systematic review and meta-analysis. Eur. Resp. J. 2006, 28, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990– 2015: A systematic analysis for the Global Burden of Disease Study (GBD 2015). Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef]
- Hang, L.-W.; Hsu, J.-Y.; Chang, C.-J.; Wang, H.-C.; Cheng, S.-L.; Lin, C.-H.; Chan, M.-C.; Wang, C.-C.; Perng, D.-W.; Yu, C.-J. Predictive factors warrant screening for obstructive sleep apnea in COPD: A Taiwan National Survey. Int. J. COPD 2016, 11, 665–673. [Google Scholar]
- Müllerova, H.; Agustí, A.; Erqou, S.; Mapel, D.W. Cardiovascular Comorbidity in COPD: Systematic Literature Review. Chest 2013, 144, 1163–1178. [Google Scholar] [CrossRef]
- McNicholas, W.T. Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea: Overlaps in Pathophysiology, Systemic Inflammation, and Cardiovascular Disease. Am. J. Respir. Crit. Care Med. 2009, 180, 692–700. [Google Scholar] [CrossRef]
- McNicholas, W.T. COPD-OSA Overlap Syndrome: evolving evidence regarding epidemiology, clinical consequences, and management. Chest 2017, 152, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Mieczkowski, B.; Ezzie, M.E. Update on obstructive sleep apnea and its relation to COPD. Int. J. COPD 2014, 9, 349–362. [Google Scholar] [Green Version]
- Lopes Zangrando, K.T.; Trimer, R.; Soares de Carvalho, L.C., Jr.; Tinoco Arêas, G.P.; Rossi Caruso, F.C.; Cabiddu, R.; Goi Roscani, M.; Galhardo Rizzatti, F.P.; Borghi-Silva, A. Chronic obstructive pulmonary disease severity and its association with obstructive sleep apnea syndrome: impact on cardiac autonomic modulation and functional capacity. Int. J. COPD 2018, 13, 1343–1351. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Messineo, L.; Perger, E.; Salameh, M.; Pini, L.; Corda, L.; Ferliga, M.; Tantucci, C. Cardiac Sympathetic Hyperactivity in Patients with Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea. COPD J. Chronic Obstr. Pulm. Dis. 2016, 13, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chen, C.; Cao, Z.; Sun, B.; Lo, I.L.; Liu, T.-M.; Zheng, J.; Sun, S.; Shi, Y.; Zhang, X.D. Entropy change of biological dynamics in COPD. Int. J. COPD 2017, 12, 2997–3005. [Google Scholar] [CrossRef] [Green Version]
- Kabbach, E.Z.; Mazzuco, A.; Borghi-Silva, A.; Cabiddu, R.; Agnoleto, A.G.; Barbosa, J.F.; Soares de Carvalho, L.C., Jr.; Gonçalves Mendes, R. Increased parasympathetic cardiac modulation in patients with acute exacerbation of COPD: how should we interpret it? Int. J. COPD 2017, 12, 2221–2230. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, A.; Schwartz, A.R.; Schneider, H.; Owens, R.L.; De Young, P.; Han, M.K.; Wedzicha, J.A.; Hansel, N.N.; Zeidler, M.R.; Wilson, K.C.; et al. On behalf of the ATS Assembly on Sleep and Respiratory Neurobiology. Research Priorities in Pathophysiology for Sleep-disordered Breathing in Patients with Chronic Obstructive Pulmonary Disease An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2018, 197, 289–299. [Google Scholar] [CrossRef]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Stein, P.K.; Pu, Y. Heart rate variability, sleep and sleep disorders. Sleep Med. Rev. 2012, 16, 47–66. [Google Scholar] [CrossRef]
- Abásolo, D.; Hornero, R.; Espino, P.; Álvarez, D.; Poza, J. Entropy analysis of the EEG background activity in Alzheimer’s disease patients. Physiol. Meas. 2006, 27, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Wu, H.T.; Chen, H.R.; Liu, A.B.; Yeh, J.J.; Lo, M.T.; Tsao, J.H.; Tang, C.-J.; Tsai, I.-T.; Sun, C.-K. Application of a Modified Entropy Computational Method in Assessing the Complexity of Pulse Wave Velocity Signals in Healthy and Diabetic Subjects. Entropy 2014, 16, 4032–4043. [Google Scholar] [CrossRef] [Green Version]
- Alcaraz, R.; Rieta, J.J. Sample entropy of the main atrial wave predicts spontaneous termination of paroxysmal atrial fibrillation. Med. Eng. Phys. 2009, 31, 917–922. [Google Scholar] [CrossRef]
- Costa, M.D.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef]
- Álvarez, D.; Hornero, R.; Marcos, J.V.; Del Campo, F. Multivariate analysis of blood oxygen saturation recordings in obstructive sleep apnea diagnosis. IEEE Trans. Biomed. Eng. 2010, 57, 2816–2824. [Google Scholar] [CrossRef]
- Gutiérrez-Tobal, G.C.; Álvarez, D.; Gomez-Pilar, J.; Del Campo, F.; Hornero, R. Assessment of time and frequency domain entropies to detect sleep apnoea in heart rate variability recordings from men and women. Entropy 2015, 17, 123–141. [Google Scholar] [CrossRef]
- Al-Angari, H.M.; Sahakian, A.V. Use of sample entropy approach to study heart rate variability in obstructive sleep apnea syndrome. IEEE Trans. Biomed. Eng. 2007, 54, 1900–1904. [Google Scholar] [CrossRef]
- Goulart, C.L.; Simon, J.C.; Schneiders, P.B.; San Martin, E.A.; Borghi-Silva, A.; Trimer, R.; de Silva, A.L. Respiratory muscle strength effect on linear and nonlinear heart rate variability parameters in COPD patients. Int. J. COPD 2016, 11, 1671–1677. [Google Scholar] [CrossRef] [Green Version]
- Borghi-Silva, A.; Mendes, R.G.; Trimer, R.; Oliveira, C.R.; Fregonezi, G.A.; Resqueti, V.R.; Arena, R.; Sampaio-Jorge, L.M.; Costa, D. Potential effect of 6 versus 12-weeks of physical training on cardiac autonomic function and exercise capacity in chronic obstructive pulmonary disease. Eur. J. Phys. Rehabil. Med. 2015, 51, 211–221. [Google Scholar]
- Dames, K.K.; Lopes, A.J.; De Melo, P.L. Airflow pattern complexity during resting breathing in patients with COPD: effect of airway obstruction. Respir. Physiol. Neurobiol. 2014, 192, 39–47. [Google Scholar] [CrossRef]
- Zamarrón, C.; Hornero, R.; Del Campo, F.; Abásolo, D.; Alvarez, D. Heart rate regularity analysis obtained from pulse oximetric recordings in the diagnosis of obstructive sleep apnea. Sleep Breath. 2006, 10, 83–89. [Google Scholar] [CrossRef]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S.F. For the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events-Rules, Terminology and Technical Specifications, 1st ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Zamarrón, C.; Gude, F.; Barcala, J.; Rodríguez, J.R.; Romero, P.V. Utility of oxygen saturation and heart rate spectral analysis obtained from pulse oximetric recordings in the diagnosis of sleep apnea syndrome. Chest 2003, 123, 1567–1576. [Google Scholar] [CrossRef]
- Redline, S.; Sanders, M.H.; Lind, B.K.; Quan, S.F.; Iber, C.; Gottlieb, D.J.; Bonekat, W.H.; Rapoport, D.M.; Smith, P.L.; Kiley, J.P. For the Sleep Heart Health Research Group. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep 1998, 21, 759–768. [Google Scholar]
- Penzel, T.; Kantelhardt, J.W.; Grote, L.; Peter, J.H.; Bunde, A. Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea. IEEE Trans. Biomed. Eng. 2003, 50, 1143–1151. [Google Scholar] [CrossRef] [Green Version]
- Goldberger, A.L. Is the normal heartbeat chaotic or homeostatic? News Physiol. Sci. 1991, 6, 87–91. [Google Scholar] [CrossRef]
- Wessel, N.; Riedl, M.; Kurths, J. Is the normal heart rate “chaotic” due to respiration? Chaos 2009, 19, 028508. [Google Scholar] [CrossRef]
- Hornero, R.; Álvarez, D.; Abásolo, D.; Del Campo, F.; Zamarrón, C. Utility of approximate entropy from overnight pulse oximetry data in the diagnosis of the obstructive sleep apnea syndrome. IEEE Trans. Biomed. Eng. 2007, 54, 107–113. [Google Scholar] [CrossRef]
- Del Campo, F.; Hornero, R.; Zamarrón, C.; Abasolo, D.E.; Álvarez, D. Oxygen saturation regularity analysis in the diagnosis of obstructive sleep apnea. Artif. Intell. Med. 2006, 37, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Tobal, G.C.; Álvarez, D.; Del Campo, F.; Hornero, R. Utility of AdaBoost to detect sleep apnea-hypopnea syndrome from single-channel airflow. IEEE Trans. Biomed. Eng. 2016, 63, 636–646. [Google Scholar] [CrossRef]
- Mondal, A.; Bhattacharyat, P.; Saha, G. Diagnosing of the lungs status using morphological anomalies of the signals in transformed domain. In Proceedings of the 4th International Conference on Intelligent Human Computer Interaction, Kharagpur, India, 27–29 December 2012. [Google Scholar]
- Garde, A.; Dehkordi, P.; Karlen, W.; Wensley, D.; Ansermino, J.M.; Dumont, G.A. Development of a screening tool for sleep disordered breathing in children using the Phone OximeterTM. PLoS ONE 2014, 9, e112959. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Pincus, S.M.; Goldberger, A.L. Physiological time series analysis: what does regularity quantify? Am. J. Physiol. 1994, 266, H1643–H1656. [Google Scholar] [CrossRef]
- Zamarrón, C.; Lado, M.J.; Teijeiro, T.; Morete, E.; Vila, X.A.; Lamas, P.F. Heart rate variability in patients with severe chronic obstructive pulmonary disease in a home care program. Technol. Health Care 2014, 22, 91–98. [Google Scholar]
- Guilleminault, C.; Winkle, R.; Connolly, S.; Melvin, K.; Tilkian, A. Cyclical variation of the heart rate in sleep apnoea syndrome: Mechanisms and usefulness of 24 h electrocardiography as a screening technique. Lancet 1984, 323, 126–131. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Romano, S.; Marrone, O.; Chiodi, M.; Bonsignore, G. Different heart rate patterns in obstructive apneas during NREM sleep. Sleep 1997, 20, 1167–1174. [Google Scholar]
- Tobaldini, E.; Nobili, L.; Strada, S.; Casali, K.R.; Braghiroli, A.; Montano, N. Heart rate variability in normal and pathological sleep. Front. Physiol. 2013, 4, 294. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Montano, N.; Cogliati, C.; Van de Borne, P.J.H.; Dyken, M.E.D.; Somers, V.K. Altered cardiovascular variability in obstructive sleep apnea. Circulation 1998, 98, 1071–1077. [Google Scholar] [CrossRef]
- Gestel, A.J.R.; Steier, J. Autonomic dysfunction in patients with chronic obstructive pulmonary disease (COPD). J. Thorac. Dis. 2010, 2, 215–222. [Google Scholar]
- Heindl, S.; Lehnert, M.; Criee, C.P.; Hasenfuss, G.; Andreas, S. Marked sympathetic activation in patients with chronic respiratory failure. Am. J. Resp. Crit. Care Med. 2001, 164, 597–601. [Google Scholar] [CrossRef]
- Da Silva, S.P.; Hulce, V.D.; Backs, R.W. Effects of obstructive sleep apnea on autonomic cardiac control during sleep. Sleep Breath. 2009, 13, 147–156. [Google Scholar] [CrossRef]
- Gula, L.J.; Krahn, A.D.; Skanes, A.; Ferguson, K.A.; George, C.; Yee, R.; et al. Heart rate variability in obstructive sleep apnea: a prospective study and frequency domain analysis. Ann. Noninvasive Electrocardiol. 2003, 8, 144–149. [Google Scholar] [CrossRef]
- Wang, W.; Tretriluxana, S.; Redline, S.; Surovec, S.; Gottlieb, D.J.; Khoo, M.C. Association of cardiac autonomic function measures with severity of sleep-disordered breathing in a community-based sample. J. Sleep Res. 2008, 17, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Mazzuco, A.; Medeiros, W.M.; Sperling, M.P.; et al. Relationship between linear and nonlinear dynamics of heart rate and impairment of lung function in COPD patients. Int. J. COPD 2015, 10, 1651–1661. [Google Scholar] [CrossRef] [Green Version]
- Iranmanesh, A.; Rochester, D.F.; Liu, J.; Veldhuis, J.D. Impaired adrenergic- and corticotropic-axis outflow during exercise in chronic obstructive pulmonary disease. Metabolism 2011, 60, 1521–1529. [Google Scholar] [CrossRef]
- Sarlabous, L.; Torres, A.; Fiz, J.A.; Jane, R. Evidence towards improved estimation of respiratory muscle effort from diaphragm mechanomyographic signals with cardiac vibration interference using sample entropy with fixed tolerance values. PLoS ONE 2014, 9, e88902. [Google Scholar] [CrossRef]
- Del Campo, F.; Hornero, R.; Zamarrón, C.; Álvarez, D.; Marcos, J.V. Variability of pulse signal frequency obtained using nocturnal pulse oximetry in patients with sleep apnoea/hypoapnoea syndrome. Arch. Bronconeumol. 2010, 46, 116–121. [Google Scholar] [CrossRef]
- Bonnemeier, H.; Wiegand, U.K.; Brandes, A.; Kluge, N.; Katus, H.A.; Richardt, G.; Potratz, J. Circadian profile of cardiac autonomic nervous modulation in healthy subjects. J. Cardiovasc. Electrophysiol. 2003, 14, 791–799. [Google Scholar] [CrossRef]
- Haarmann, H.; Mohrlang, C.; Tschiesner, U.; Rubin, D.B.; Bornemann, T.; Rüter, K.; Bonev, S.; Raupach, T.; Hasenfuß, G.; Andreas, S. Inhaled β-agonist does not modify sympathetic activity in patients with COPD. BMC Pulm. Med. 2015, 15, 46. [Google Scholar] [CrossRef]
- Wu, Y.K.; Huang, C.Y.; Yang, M.C.; Huang, G.L.; Chen, S.Y.; Lan, C.C. Effect of tiotropium on heart rate variability in stable chronic obstructive pulmonary disease patients. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 100–105. [Google Scholar] [CrossRef]


| Demographics | COPD | OSAS | COPD + OSAS | p-Value |
|---|---|---|---|---|
| Nº of subjects | 22 | 213 | 62 | - |
| Nº of males (%) | 16 (72.7%) | 167 (78.4%) | 58 (93.6%) | - |
| Age (years) | 60.5 (57, 64) ¶ | 56 (47, 64) † | 66 (60, 75) ¶† | <0.05 |
| BMI (kg/m2) | 27.3 (24.3, 29.4) | 29.4 (26.7, 32.8) | 29.6 (26.8, 32.7) | N.S. |
| Polysomnography | ||||
| AHI (events/h) | 9.0 (5.2, 11.1) *¶ | 44.2 (30.6, 66.5) * | 53.7 (29.0, 67.2) ¶ | <0.05 |
| ODI3 (events/h) | 9.9 (5.3, 11.4) *¶ | 42.7 (30.6, 62.4) * | 51.6 (31.7, 64.4) ¶ | <0.05 |
| CT90 (%) | 22.9 (0.4, 87.3) ¶ | 14.3 (6.3, 39.7) † | 47.8 (24.7, 88.2) ¶† | <0.05 |
| SpO2basal (%) | 92 (90, 94) * | 94 (93, 94) *† | 91 (90, 93) † | <0.05 |
| SpO2min (%) | 85 (83, 87) *¶ | 80 (73, 83) *† | 76.5 (71, 81) ¶† | <0.05 |
| SpO2avg (%) | 91.5 (89, 94) ¶ | 92 (90, 93) † | 90 (88, 92) ¶† | <0.05 |
| Pulmonary Function (Spirometry) | ||||
| FVC (liters) | 3.2 (2.3, 3.8) | N.A. | 2.6 (2.2, 3.5) | N.S. |
| FVC (%) | 91 (72.8, 100) | N.A. | 77.3 (67, 92) | <0.05 |
| FEV1 (liters) | 1.9 (1.5, 2.2) | N.A. | 1.6 (1.3, 2.1) | N.S. |
| FEV1 (%) | 68.5 (55.0, 83.2) | N.A. | 62.5 (54.3, 73.0) | N.S. |
| FEV1/FVC | 59.4 (50.1, 65.2) | N.A. | 60.9 (52.0, 65.4) | N.S. |
| FVC improvement | 3.3 (2.6, 11.0) | N.A. | 3.9 (2.5, 7.3) | N.S. |
| FEV1 improvement | 3.9 (2, 8) | N.A. | 3 (1.6, 5.3) | N.S. |
| COPD (22) | OSAS (213) | COPD+OSAS (62) | p-Value | |
|---|---|---|---|---|
| Comorbidities | ||||
| Hypertension, n (%) | 10 (45.5%) | 88 (41.3%) † | 42 (67.7%) † | <0.05 |
| Ischemic Cardiomyopathy, n (%) | 2 (9.1%) | 11 (5.2%) † | 10 (16.1%) † | <0.05 |
| Diabetes, n (%) | 3 (13.6%) | 22 (10.3%) | 9 (14.5%) | N.S. |
| Medications | ||||
| Beta-blockers, n (%) | 2 (9.1%) | 25 (11.7%) † | 15 (24.2%) † | <0.05 |
| Calcium antagonists, n (%) | 1 (4.5%) | 18 (8.5%) | 6 (9.7%) | N.S. |
| Anticholinergics, n (%) | 8 (36.4%) | 1 (0.5%) | 36 (58.1%) | N.S. ǂ |
| Beta2-Agonists, n (%) | 8 (36.4%) | 8 (3.8%) | 29 (46.8%) | N.S. ǂ |
| Inhaled corticosteroids, n (%) | 7 (31.8%) | 9 (4.2%) | 27 (43.6%) | N.S. ǂ |
| COPD | OSAS | COPD + OSAS | p-Value | |
|---|---|---|---|---|
| Time domain analysis | ||||
| AVNN | 0.873 (0.825, 0.980) | 0.961 (0.852, 1.051) † | 0.906 (0.787, 1.022) † | 0.026 (p < 0.05) |
| SDNN | 0.066 (0.058, 0.074) | 0.075 (0.062, 0.094) | 0.071 (0.056, 0.090) | 0.088 (N.S.) |
| RMSSD (10−4) | 0.577 (0.466, 0.756) | 0.724 (0.578, 0.863) | 0.686 (0.522, 0.831) | 0.071 (N.S.) |
| Frequency Domain Analysis | ||||
| VLFn | 0.802 (0.774, 0.825) | 0.821 (0.792, 0.844) | 0.812 (0.772, 0.844) | 0.061 (N.S.) |
| LFn | 0.938 (0.931, 0.961) | 0.951 (0.934, 0.962) † | 0.941 (0.930, 0.956) † | 0.028 (p < 0.05) |
| HFn | 0.062 (0.039, 0.069) | 0.049 (0.038, 0.066) † | 0.059 (0.044, 0.070) † | 0.028 (p < 0.05) |
| LF/HF | 15.243 (13.569, 24.926) | 19.419 (14.179, 25.343) † | 15.881 (13.301, 21.673) † | 0.028 (p < 0.05) |
| OSASFn | 0.282 (0.240, 0.302) *¶ | 0.308 (0.274, 0.351) * | 0.310 (0.268, 0.357) ¶ | 0.022 (p < 0.05) |
| Nonlinear Analysis | ||||
| SampEn | 0.212 (0.151, 0.267) ¶ | 0.241 (0.189, 0.325) † | 0.267 (0.210, 0.407) ¶† | 0.022 (p < 0.05) |
| Author | Population | Aim | HRV Acquisition Setting | Non-Linear Analysis | Findings |
|---|---|---|---|---|---|
| Zamarrón et al. (2006) [26] | 187 suspicion OSAS:
| PRV assessment in OSAS patients | Night-time while sleeping (attended) | ApEn | Increased irregularity (ApEn) in overlap patients |
| Borghi-Silva et al. (2015) [24] | 20 COPD | Effect of physical training on HRV in COPD | Daytime at rest | SampEn | HRV irregularity increases after physical training |
| Mazzuco et al. (2015) [50] | 16 COPD | HRV assessment in increasing COPD severity | Daytime at rest | ApEn | HRV irregularity decreases during RSA |
| Goulart et al. (2016) [23] | 10 COPD | HRV assessment in COPD | Daytime at rest | SampEn and ApEn | HRV irregularity decreases during RSA |
| Kabbach et al. (2017) [12] | 32 COPD:
| HRV assessment in COPD with and without exacerbation | Daytime at rest | ApEn, SampEn, and scatter plots | Irregularity (entropy) decreases while variability (dispersion) increases after exacerbation |
| Zangrando et al. (2018) [9] | 24 COPD:
| HRV assessment in COPD | Daytime at rest | Scatter plots | Higher (N.S.) variability in overlap patients |
| Current study (2019) | 297 suspicion OSAS:
| PRV assessment in COPD patients with and without OSAS | Night-time while sleeping (unattended) | SampEn | Increased irregularity (SampEn) in overlap patients |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, D.; Sánchez-Fernández, A.; Andrés-Blanco, A.M.; Gutiérrez-Tobal, G.C.; Vaquerizo-Villar, F.; Barroso-García, V.; Hornero, R.; del Campo, F. Influence of Chronic Obstructive Pulmonary Disease and Moderate-To-Severe Sleep Apnoea in Overnight Cardiac Autonomic Modulation: Time, Frequency and Non-Linear Analyses. Entropy 2019, 21, 381. https://doi.org/10.3390/e21040381
Álvarez D, Sánchez-Fernández A, Andrés-Blanco AM, Gutiérrez-Tobal GC, Vaquerizo-Villar F, Barroso-García V, Hornero R, del Campo F. Influence of Chronic Obstructive Pulmonary Disease and Moderate-To-Severe Sleep Apnoea in Overnight Cardiac Autonomic Modulation: Time, Frequency and Non-Linear Analyses. Entropy. 2019; 21(4):381. https://doi.org/10.3390/e21040381
Chicago/Turabian StyleÁlvarez, Daniel, Ana Sánchez-Fernández, Ana M. Andrés-Blanco, Gonzalo C. Gutiérrez-Tobal, Fernando Vaquerizo-Villar, Verónica Barroso-García, Roberto Hornero, and Félix del Campo. 2019. "Influence of Chronic Obstructive Pulmonary Disease and Moderate-To-Severe Sleep Apnoea in Overnight Cardiac Autonomic Modulation: Time, Frequency and Non-Linear Analyses" Entropy 21, no. 4: 381. https://doi.org/10.3390/e21040381





