Transfer Information Assessment in Diagnosis of Vasovagal Syncope Using Transfer Entropy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Data Collections
2.3. Data Analysis and Statistical Methods
2.4. Transfer Entropy
3. Results
3.1. Comparisons of Transfer Entropy between Different Tilt Test Phases for HUTT(+) and HUTT(-) Group
3.2. Comparisons of TE between HUTT(-) versus HUTT(+) Group in Individual Phases of HUTT
4. Discussion
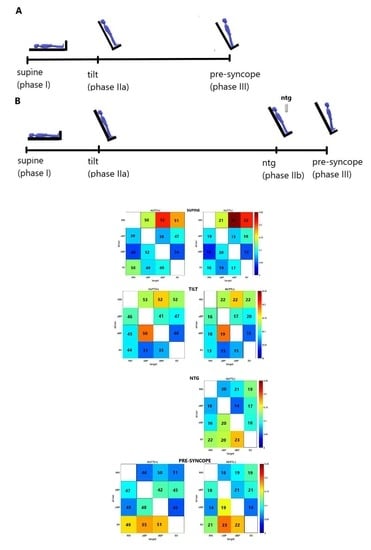
- in supine position (phase I): TE was greater than 0 in both groups for all driver-target pairs. The highest values of TE were noted for RRI-dBP and RRI-SV (Figure 3a) and lightly lower TE was observed for the RRI-SV link.
- in response to the tilt (phase IIa): highest values of TE were also found in both groups for the RRI-dBP links and slightly lower for: RRI-sBP and RRI-SV (Figure 3b). In the HUTT(+) group we noted a high value of TE(dBP-sBP).
- in the phase IIb (nitroglycerin administration): HUTT(-) manifested the highest value of TE for the SV driver to the following targets: RRI, sBP and dBP (Figure 3c).
- in the pre-syncope phase (III): the highest information transfer in both groups was related to the SV driver in conjunction with RRI, sBP and dBP as targets (Figure 3d). In both groups, the highest was TE(SV-sBP) and slightly lower TE SV-dBP) and T (SV-RRI).
- in the HUTT(-) group there were significant differences between the following phases: supine vs. pre-syncope (I vs. III) and tilt vs. pre-syncope (IIa vs. III) for each target (TE(RRI-sBP), TE(RRI-dBP) and TE(RRI-SV): p < 0.05).
- Similar outcome was noted in HUTT(+) patients for TE(RRI-dBP). Significant differences between supine and pre-syncope were also observed for TE(RRI-sBP) (Figure 2a).
- we did not observe significant differences in both groups between HUT phases (Figure 2b).
- statistically significant differences were noted only in the HUTT(-) group for TE(dBP-sBP). The value of TE(dBP-sBP) in phase IIa (tilt) increased significantly in comparison with phase I (supine) from 0.08 to 0.18. There were also significant differences between supine and pre-syncope (I vs. II).
- In HUTT(-) patients there were no significant differences (Figure 2c).
- In both groups the values of TE(SV-RRI), TE(SV-sBP), TE(SV-dBP) significantly increased between tilt and pre-syncope (IIa vs. III). The occurrence of syncope after the tilt is associated with the increasing information transfer from SV to all components of the observed system.
- In the HUTT(-) group the significant increasing of TE(SV-sBP) and TE(SV-dBP) was observed between the ntg administration and pre-syncope (IIb vs. III).
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TLOC | transient loss of consciousness |
| TE | transfer entropy |
| TE (driver-target) | transfer entropy form driver to target |
| VVS | Vasovagal Syndrome |
| HUTT | head up tilt test |
| HUTT(+) | patients with syncope during the passive phase of HUTT |
| HUTT(−) | patients without syncope during the passive phase of HUTT, requiring pharmacological provocation with nitroglycerine (ntg) |
| RRI | R-R intervals |
| sBP | systolic blood pressure |
| dBP | diastolic blood pressure |
| SV | stroke volume |
References
- Brignole, M.; Moya, A.; de Lange, F.; Deharo, J.-C.; Elliot, P.M.; Faniculli, A. Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 30, 2631–2671. [Google Scholar]
- Parry, S.W.; Reeve, P.; Lawson, J.; Shaw, F.E.; Davison, J.; Norton, M.; Frearson, R.; Kerr, S.; Newton, J.L. The Newcastle Protocols 2008: An update on head-up tilt table testing and the management of vasovagal syncope and related disorders. Heart 2009, 95, 416–420. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, J.J.; Wieling, W.; Karemaker, J.M.; Eckberg, D. The vasovagal response. Clin. Sci. 1991, 81, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Oberg, B.; Thoren, P. Increased activity in the A ventricular receptors during hemorrhage or occlusion of caval veins in the cat: A possible cause of vasovagal reaction. Acta Physiol. Scand. 1872, 85, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Montalto, A.; Faes, L.; Marinazzo, D. MuTE: A MATLAB Toolbox to Compare Established and Novel Estimators of the Multivariate Transfer Entropy. PLoS ONE 2014, 9, e109462. [Google Scholar] [CrossRef] [PubMed]
- Wibral, M.; Rahm, B.; Rieder, M.; Lindner, M.; Vicente, R.; Kaiser, J. Transfer entropy in magnetoencephalographic data: Quantifying information flow in cortical and cerebellar networks. Progr. Biophys. Mol. Biol. 2011, 105, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Wibral, M.; Lindner, M.; Pipa, G. Transfer entropy-a model-free measure of effective connectivity for the neurosciences. J. Comp. Neurosci. 2011, 30, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Vakorin, V.A.; Kovacevic, N.; McIntosh, A.R. Exploring transient transfer entropy based on a group-wise ICA decomposition of EEG data. Neuroimage 2010, 49, 1593–1600. [Google Scholar] [CrossRef]
- Gourevitch, B.; Eggermont, J.J. Evaluating information transfer between auditory cortical neurons. J. Neurophysiol. 2007, 97, 2533–2543. [Google Scholar] [CrossRef]
- Faes, L.; Nollo, G.; Porta, A. Information domain approach to the investigation of cardio-vascular, cardio-pulmonary, and vasculo-pulmonary causal couplings. Front. Physiol. 2011, 2, 1–13. [Google Scholar] [CrossRef]
- Faes, L.; Nollo, G.; Porta, A. Non-uniform multivariate embedding to assess the information transfer in cardiovascular and cardiorespiratory variability series. Comput. Biol. Med. 2012, 42, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Faes, L.; Nollo, G.; Porta, A. Compensated Transfer Entropy as a Tool for Reliably Estimating Information Transfer in Physiological Time Series. Entropy 2013, 15, 198–216. [Google Scholar] [CrossRef]
- Porta, A.; Marchi, A.; Bari, V.; De Maria, B.; Esler, M.; Lambert, E.; Baumert, M. Assessing the strength of cardiac and sympathetic baroreflex controls via transfer entropy during orthostatic challenge. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375, 2096. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Catai, A.M.; Takahashi, A.C.; Magagnin, V.; Bassani, T.; Tobaldini, E.; Montano, N. Information transfer through the spontaneous baroreflex in healthy humans. Methods Inf. Med. 2010, 49, 506–510. [Google Scholar] [PubMed]
- Wejer, D.; Faes, L.; Makowiec, D. Causal relationships in the variability of cardiovascular system evoked by orthostatic stress by transfer entropy. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 3799–3802. [Google Scholar] [CrossRef]
- Graff, B.; Wejer, D.; Faes, L.; Graff, G.; Makowiec, D.; Narkiewicz, K. The use of Transfer Entropy method for the assessment of cardiovascular regulation during head-up tilt test. Autonomic Neurosci. Basic Clin. 2015, 192, 101–102. [Google Scholar] [CrossRef]
- Buszko, K.; Piątkowska, A.; Koźluk, E.; Fabiszak, T.; Opolski, G. Entropy Measures in Analysis of Head up Tilt Test Outcome for Diagnosing Vasovagal Syncope. Entropy 2018, 20, 976. [Google Scholar] [CrossRef]
- Brignole, M.; Menozzi, C.; Del Rosso, A.; Costa, S.; Gaggioli, G.; Bottoni, N.A.; Sutton, R. New classification of hemodynamics of vasovagal syncope: Beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace 2000, 2, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Task Force® Monitor. Available online: https://www.cnsystems.com/products/task-force-monitor (accessed on 10 January 2019).
- Schwalm, T. Modern Tilt Table Testing and Non-Invasive Monitoring; ABW Wissenschftsverlag GmbH: Berlin, Germany, 2007. [Google Scholar]
- Verheyden, B.; Ector, H.; Aubert, A.E.; Reybrouck, T. Tilt training increases the vasoconstrictor reserve in patients with neurally mediated syncope evoked by head-up tilt testing. Eur. Heart J. 2008, 29, 1523–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, A.P.; Theodorakis, G.; Vardas, P.; Sutton, R. Methodology of head-up tilt testing in patients with unexplained syncope. J. Am. Coll. Cardiol. 1991, 17, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Montalto, Alessandro (2015): MuTE. figshare. Code. Available online: http://dx.doi.org/10.6084/m9.figshare.1005245 (accessed on on 1 December 2018).
- Tort, A.B.L.; Komorowski, R.; Eichenbaum, H.; Kopell, N. Measuring Phase-Amplitude Coupling Between Neuronal Oscillations of Different Frequencies. J. Neurophysiol. 2010, 104, 1195–1210. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Ji, L.; Yan, C.; Li, K.; Liu, C.; Liu, C. Coupling between short-term heart rate and diastolic period is reduced in heart failure patients as indicated by multivariate entropy analysis. Comput. Cardiol. 2014, 41, 97–100. [Google Scholar]
- Li, P.; Liu, C.; Sun, X.; Ren, Y.; Yan, C.; Yu, Z.; Liu, C. Age related changes in variability of short term heart rate and diastolic period. Comput. Cardiol. 2013, 40, 995–998. [Google Scholar]
- Liu, C. Measuring synchronization in coupled simulation and coupled cardiovascular time series: A comparison of different cross entropy. Biomed. Signal Proc. Control. 2015, 21, 49–57. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Wang, X.; Li, L.; Yang, L.; Chen, Y.; Liu, C. Testing pattern synchronization in coupled system througth different entropy-based measures. Med. Biol. Eng. Comput. 2013, 51, 581–591. [Google Scholar]
- Xie, H.B.; Guo, J.Y.; Zheng, Y.P. A comparative study of pattern synchronization detection between neural signals using different cross-entropy measures. Biol. Cybern. 2010, 102, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y. Is cross- sample entropya valid measure of synchronization between sequences of RR interval and pulse transit time? Comput. Cardiol. 2013, 40, 939–942. [Google Scholar]
- Li, P.; Liu, C.; Wang, X.; Li, B.; Che, W.; Liu, C. Cross-sample entropy and cross-fuzzy entropyfor testing pattern synchrony: How results vary with different threshold value r. Word. Congr. Med. Phys. Biomed. Eng. 2012, 39, 485–488. [Google Scholar]
- Pincus, S. Approximate entropy as a complexity measure. Chaos 1995, 5, 10–117. [Google Scholar] [CrossRef]
- Schreiber, T. Measuring information transfer. Phys. Rev. Lett. 2000, 85, 461. [Google Scholar] [CrossRef] [PubMed]
- Makowiec, D.; Wejer, D.; Graff, B.; Struzik, Z.R. Dynamical Pattern Representation of Cardiovascular Couplings Evoked by Head-up Tilt Test. Entropy 2018, 20, 235. [Google Scholar] [CrossRef]
- Javorka, M.; Krohova, J.; Czippelova, B.; Turianikova, Z.; Lazarova, Z.; Wiszt, R.; Faes, L. Towards understanding the complexity of cardiovascular oscillations: Insights from information theory. Comput. Biol. Med. 2018, 98, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, M.; Pini, N.; Fifer, W.P.; Burtchen, N.; Signorini, M.G. Entropy Information of Cardiorespiratory Dynamics in Neonates during Sleep. Entropy 2017, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Pan, W.; Li, Y.; Feng, K.; Liu, G. The Interaction Analysis between the Sympathetic and Parasympathetic Systems in CHF by UsingTransfer Entropy Method. Entropy 2018, 20, 795. [Google Scholar] [CrossRef]
- Zheng, L.; Pan, W.; Li, Y.; Luo, D.; Wang, Q.; Liu, G. Use of Mutual Information and Transfer Entropy to Assess Interaction between Parasympathetic and Sympathetic Activities of Nervous System from HRV. Entropy 2017, 19, 489. [Google Scholar] [CrossRef]
- Buszko, K.; Piątkowska, A.; Koźluk, E.; Opolski, G. Entropy in investigation o f vasovagal syndrome in passive head up tilt test. Entropy 2017, 19, 236. [Google Scholar] [CrossRef]
- Buszko, K.; Piatkowska, A.; Koźluk, E.; Fabiszak, T.; Opolski, G. The complexity of hemodynamic response to the tilt test with and without nitroglycerine provocation in patients with vasovagal syncope. Sci. Rep. 2018, 8, 15554. [Google Scholar] [CrossRef]
- Buszko, K.; Kujawski, S.; Newton, J.L.; Zalewski, P. Hemodynamic response to the head-up tilt test in patients with syncope as a predictor of the test outcome: A meta-analysis approach. Front. Physiol. 2019, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Koźluk, E.; Cybulski, G.; Piątkowska, A.; Zastawna, I.; Niewiadomski, W.; Strasz, A.; Gąsiorowska, A.; Kempa, M.; Kozłowski, D.; Opolski, G. Early hemodynamic response to the tilt test in patients with syncope. Clin. Res. 2014, 10, 1078–1085. [Google Scholar] [CrossRef] [Green Version]






| Baseline | HUTT(+) (n = 57: F = 43, M = 14) | HUTT(-) (n = 23: F = 17, M = 6) |
|---|---|---|
| Female-age [y] | 35.6 ± 16 | 32.3 ± 12 |
| Male-age [y] | 41.7 ± 15.6 | 43 ± 15 |
| HR [bpm] | 72.06 ± 9.98 | 66.1 ± 16.8 |
| sBP [mmHg] | 108.6 ± 25.4 | 102.6 ± 20.4 |
| dBP [mmHg] | 68.0 ± 19.7 | 66.1 ± 16.8 |
| Hypertension | 3 | 0 |
| Diabetes | 0 | 0 |
| Medication | 2 | 4 |
| Transfer Entropy TE (Driver-Target) | Phase I (Supine) | Phase IIa (Tilt) | Phase III (Pre-Syncope) |
|---|---|---|---|
| mean ± std median [IQR] | mean ± std median [IQR] | mean ± std median [IQR] | |
| TE (RRI-sBP) | 0.14 ± 0.12 | 0.13 ± 0.09 | 0.07 ± 0.08 |
| 0.10 [0.13] | 0.11 [0.10] | 0.04 [0.08] | |
| TE (RRI-dBP) | 0.20 ± 0.18 | 0.16 ± 0.11 | 0.09 ± 0.09 |
| 0.15 [0.21] | 0.15 [0.13] | 0.07 [0.09] | |
| TE(RRI-SV) | 0.17 ± 0.15 | 0.13 ± 0.12 | 0.11 ± 0.10 |
| 0.15 [0.20] | 0.10 [0.12] | 0.07 [0.12] | |
| TE (sBP-RRI) | 0.08 ± 0.10 | 0.10 ± 0.10 | 0.10 ± 0.13 |
| 0.15 [0.08] | 0.07 [0.16] | 0.05 [0.12] | |
| TE (sBP-dBP) | 0.07 ± 0.10 | 0.10 ± 0.12 | 0.07 ± 0.09 |
| 0.03 [0.20] | 0.07 [0.18] | 0.05 [0.10] | |
| TE(sBP-SV) | 0.09 ± 0.11 | 0.09 ± 0.09 | 0.09 ± 0.10 |
| 0.06 [0.09] | 0.08 [0.09] | 0.07 [0.14] | |
| TE (dBP-RRI) | 0.04 ± 0.05 | 0.08 ± 0.09 | 0.06 ± 0.08 |
| 0.03 [0.20] | 0.06 [0.13] | 0.03 [0.08] | |
| TE(dBP-sBP) | 0.09 ± 0.11 | 0.19 ± 0.19 | 0.15 ± 0.15 |
| 0.05 [0.08] | 0.13 [0.23] | 0.11 [0.17] | |
| TE(dBP-SV) | 0.04 ± 0.06 | 0.05 ± 0.05 | 0.09 ± 0.10 |
| 0.02 [0.05] | 0.03 [0.08] | 0.04 [0.06] | |
| TE (SV-RRI) | 0.12 ± 0.13 | 0.08 ± 0.10 | 0.12 ± 0.11 |
| 0.09 [0.15] | 0.05 [0.12] | 0.10 [0.12] | |
| TE (SV-sBP) | 0.08 ± 0.09 | 0.05 ± 0.07 | 0.19 ± 0.12 |
| 0.05 [0.07] | 0.02 [0.07] | 0.18 [0.17] | |
| TE (SV-dBP) | 0.07 ± 0.10 | 0.07 ± 0.08 | 0.17 ± 0.13 |
| 0.06 [0.13] | 0.04 [0.11] | 0.14 [0.19] |
| Transfer Entropy TE (driver-target) | Phase I (Supine) | Phase IIa (Tilt) | Phase IIb (Nt) | Phase III (Pre-Syncope) |
|---|---|---|---|---|
| mean ± std median [IQR] | mean ± std median [IQR] | mean ± std median [IQR] | mean ± std median [IQR] | |
| TE (RRI-sBP) | 0.14 ± 0.12 | 0.15 ± 0.08 | 0.07 ± 0.06 | 0.05 ± 0.05 |
| 0.09 [0.17] | 0.09 [0.17] | 0.07 [0.08] | 0.04 [0.07] | |
| TE (RRI-dBP) | 0.25 ± 0.22 | 0.18 ± 0.12 | 0.08 ± 0.09 | 0.07 ± 0.08 |
| 0.18 [0.26] | 0.16 [0.16] | 0.05 [0.09] | 0.05 [0.08] | |
| TE(RRI-SV) | 0.20 ± 0.18 | 0.14 ± 0.12 | 0.12 ± 0.14 | 0.06 ± 0.06 |
| 0.09 [0.17] | 0.09 [0.17] | 0.07 [0.08] | 0.04 [0.07] | |
| TE (sBP-RRI) | 0.09 ± 0.11 | 0.09 ± 0.09 | 0.07 ± 0.08 | 0.09 ± 0.08 |
| 0.05 [0.07] | 0.07 [0.14] | 0.04 [0.10] | 0.07 [0.17] | |
| TE (sBP-dBP) | 0.07 ± 0.08 | 0.10 ± 0.11 | 0.05 ± 0.07 | 0.11 ± 0.11 |
| 0.06 [0.12] | 0.07 [0.20] | 0.07 [0.08] | 0.10 [0.10] | |
| TE(sBP-SV) | 0.10 ± 0.11 | 0.06± 0.06 | 0.10± 0.09 | 0.05 ± 0.05 |
| 0.09 [0.11] | 0.06 [0.06] | 0.07 [0.08] | 0.08 [0.12] | |
| TE (dBP-RRI) | 0.03 ± 0.04 | 0.11 ± 0.10 | 0.09 ± 0.10 | 0.06 ± 0.09 |
| 0.02 [0.06] | 0.06 [0.13] | 0.07 [0.08] | 0.03 [0.08] | |
| TE (dBP-sBP) | 0.09 ± 0.13 | 0.12 ± 0.14 | 0.13 ± 0.15 | 0.10 ± 0.12 |
| 0.05 [0.08] | 0.13 [0.23] | 0.08 [0.13] | 0.11 [0.17] | |
| TE(dBP-SV) | 0.04 ± 0.08 | 0.06± 0.08 | 0.09 ± 0.09 | 0.05 ± 0.05 |
| 0.02 [0.05] | 0.03 [0.08] | 0.06 [0.15] | 0.04 [0.06] | |
| TE (SV-RRI) | 0.09 ± 0.08 | 0.08± 0.12 | 0.13 ± 0.08 | 0.16±0.12 |
| 0.06 [0.16] | 0.02 [0.16] | 0.13 [0.12] | 0.13 [0.19] | |
| TE (SV-sBP) | 0.06 ± 0.04 | 0.06± 0.06 | 0.14 ± 0.11 | 0.18±0.10 |
| 0.06 [0.10] | 0.04 [0.13] | 0.22 [0.17] | 0.16 [0.16] | |
| TE (SV-dBP) | 0.09 ± 0.09 | 0.08 ± 0.09 | 0.17 ±0.09 | 0.17±0.12 |
| 0.04 [0.17] | 0.04 [0.12] | 0.17 [0.14] | 0.13 [0.18] |
| HUTT(-) vs. HUTT(+) | ||||||
|---|---|---|---|---|---|---|
| TE(Driver-Target) | Phase I (Supine) | Adjusted p | Phase IIa (Tilt) | Adjusted p | Phase III (Pre-Syncope) | Adjusted p |
| TE (RRI-sBP) | 1 | 1 | 1 | |||
| TE (RRI-dBP) | 1 | 1 | 1 | |||
| TE(RRI-SV) | 1 | 1 | 0.135 | |||
| TE (sBP-RRI) | 1 | 1 | 1 | |||
| TE (sBP-dBP) | 1 | 1 | 0.27 | |||
| TE(sBP-SV) | 1 | 1 | 1 | |||
| TE (dBP-RRI) | 1 | 0.87 | 1 | |||
| TE(dBP-sBP) | 1 | 0.27 | 0.8 | |||
| TE(dBP-SV) | 1 | 1 | 1 | |||
| TE (SV-RRI) | 1 | 0.87 | 0.57 | |||
| TE (SV-sBP) | 1 | 1 | 1 | |||
| TE (SV-dBP) | 1 | 1 | 1 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buszko, K.; Piątkowska, A.; Koźluk, E.; Fabiszak, T.; Opolski, G. Transfer Information Assessment in Diagnosis of Vasovagal Syncope Using Transfer Entropy. Entropy 2019, 21, 347. https://doi.org/10.3390/e21040347
Buszko K, Piątkowska A, Koźluk E, Fabiszak T, Opolski G. Transfer Information Assessment in Diagnosis of Vasovagal Syncope Using Transfer Entropy. Entropy. 2019; 21(4):347. https://doi.org/10.3390/e21040347
Chicago/Turabian StyleBuszko, Katarzyna, Agnieszka Piątkowska, Edward Koźluk, Tomasz Fabiszak, and Grzegorz Opolski. 2019. "Transfer Information Assessment in Diagnosis of Vasovagal Syncope Using Transfer Entropy" Entropy 21, no. 4: 347. https://doi.org/10.3390/e21040347