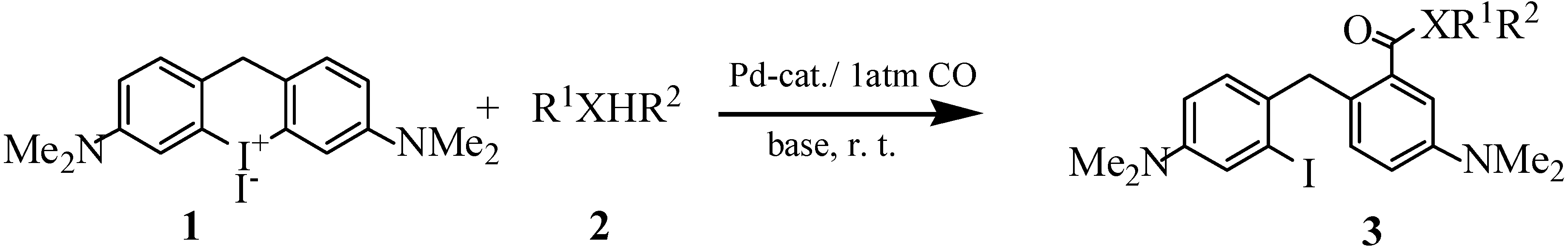General Procedure for the Synthesis of Compounds 3a, 3b, 3d and 3e.
Compound 1 (506mg, 1 mmol), palladium acetate (0.02 mmol) and methanol (20 mL) were mixed under an argon atmosphere at room temperature. After adding Bu3N (2 mmol), the inflow of argon was stopped and this gas was replaced with CO (1 atm) and the yellow suspension was stirred for 2h until a clear solution was obtained. The reaction was quenched with aqueous saturated NH4Cl solution, then the mixture was extracted three times with Et2O and the combined ether layers were dried over anhydrous MgSO4. After evaporating the solvent, the crude product was separated by flash chromatography on silica gel using Et2O and petroleum ether as eluents to give methyl 2-[4’-(dimethylamino)- 2’-iodobenzyl]-4-dimethylaminobenzoate (3a) as yellow crystals (355 mg, 81%); m.p. 91-92oC; IR: ν = 3085, 1718, 1603, 1504, 583 cm-1; 1H-NMR: δ = 2.90 (s, 6H), 2.98 (s, 2H), 3.66 (s, 3H), 4.25 (s, 2H), 6.65-7.33 (m, 6H) ppm; MS: m/z = 438 (76) [M+], 406 (22), 379 (14), 279 (76), 252 (28).
Similarly the following compounds were prepared:
Ethyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoate (3b). Yellow crystals (353 mg, 78%); m.p. 85-87 oC; IR: ν = 3075, 1713, 1602, 1500, 582 cm-1; 1H-NMR: δ = 0.94 (d, J=5.6 Hz, 3H), 2.79 (s, 6H), 2.88 (s, 6H), 4.16 (q, J=5.6Hz, 2H), 4.26 (s, 2H), 6.62 – 7.13 (m, 6H) ppm; MS: m/z = 452 (100) [M+], 423 (19), 406 (39), 325 (72), 279 (88).
n-Propyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoate (3d). Yellow crystals (326 mg, 70%); m.p. 64-65 oC; IR: ν = 3075, 2964, 1713, 1605, 1504, 582 cm-1; 1H-NMR: δ = 1.00 (t, J=7.1 Hz, 3H), 1.79 (m, J=6.9 Hz, 2H), 2.91 (s, 6H), 2.98 (s, 6H), 4.26 (q, J=6.2 Hz, 4H), 6.66 – 7.36 (m, 6H) ppm; MS: m/z = 466 (100) [M+], 423 (28), 406 (50), 339 (37), 279 (48), 452, 423 (19), 406 (39), 325 (72), 279 (88).
Diethylaminoethyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoate (3e). Viscous orange liquid (330 mg, 65%); IR: ν = 3020, 2967, 1715, 1604, 1504, 580, 443 cm-1; 1H-NMR: δ = 1.10 (t, J=7.1 Hz, 6H), 2.76 (m, 6H), 4.21 (s, 2H), 4.38 (t, J=6.3 Hz, 2H), 6.65 – 7.29 (m, 6H) ppm; MS: m/z = 523 [M+] (59), 424 (86), 406 (50), 339 (13), 379 (13), 280 (100).
General Procedure for the Synthesis of Compounds 3f, 3g and 3o.
Compound 1 (1 mmol), palladium acetate (0.02 mmol), DMF (15 mL), Bu3N (2 mmol) and n-propylamine (2 mmol) were added successively under an argon atmosphere and mixed at room temperature. The temperature was raised to 40oC, the argon flow was stopped and this gas was replaced by CO (1 atm.) and stirring was continued until the yellow suspension turned into a clear solution. After reacting for 15 min. the reaction mixture was cooled to room temperature and worked up as described for 3a to give N-n-propyl-2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethyl- aminobenzoyl amide (3f) as yellow crystals (419 mg, 90%); m.p. 139-140 oC; IR: ν = 1667, 1543 cm-1; 1H-NMR: δ = 0.96 (t, 3H), 1.58 (m, 2H), 2.89 (s, 6H), 2.97 (s, 6H), 3.32 (q, 2H), 4.09 (s, 2H), 6.65 (d, J=2.46 Hz, 1H), 6.73 (s, 1H), 6.86 (d, J=2.46 Hz, 1H), 6.94 (s, 1H), 7.20 (d, J=2.37 Hz, 1H), 7.33 (d, J=2.37 Hz, 1H) ppm; MS: m/z = 465 [M+] (8), 436 (60), 406 (19), 279 (100).
The following compounds were similarly prepared:
N-n-Butyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoyl amide (3g). Yellow crystals; (434 mg, 91%); m.p. 118-120 oC; IR: ν = 1664, 1553 cm-1; 1H-NMR: δ = 0.96 – 1.03 (m, 3H), 1.23 – 1.67 (m, 4H), 2.90 (s, 6H), 2.97 (s, 6H), 3.25 – 3.55 (m, 2H), 4.08 (s, 2H), 6.65 (d, J=2.56 Hz, 1H), 6.73 (s, 1H), 6.87 (d, J=2.56 Hz, 1H), 6.94 (s, 1H), 7.20 (d, J=2.35 Hz, 1H), 7.33 (d, J=2.35 Hz, 1H) ppm; MS: m/z = 479 [M+] (12), 436 (62), 406 (15), 279 (100).
N,N-Diethyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoyl amide (3o). Yellow crystals (311 mg, 65%); m.p. 88-89 oC; IR: ν = 1675, 1637, 1603, 1504 cm-1; 1H-NMR: δ = 1.00 – 1.36 (m, 6H), 2.91 (s, 6H), 2.95 (s, 6H), 3.22 (q, 2H), 3.54 (q, 2H), 4.34 (s, 2H), 6.58 – 6.93 (m, 4H), 7.15 – 7.28 (m, 2H) ppm; MS: m/z = 479 [M+] (10.8), 406 (85.8), 352 (17.1), 280 (91.9), 279 (100).
General Procedure for the Synthesis of Compounds 3h-3n.
The method was as described for the preparation of 3f, but n-propylamine was replaced by otheramines, i.e. phenylamine (2h), p-methylphenylamine(2i), m-methylphenylamine (2j), m-ethoxy- phenylamine (2k), o-chlorophenylamine (2l), 2-aminobenzothiazole (2m), N-methylphenylamine (2 mmol) and the reaction temperature was 70 oC rather than 40 oC. In this manner the following compounds were prepared:
N-Phenyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoyl amide (3h). Red crystals (444 mg, 89%); m.p. 197-198 oC; IR: ν = 1664, 1562 cm-1; 1H-NMR: δ = 2.94 (s, 6H), 2.96 (s, 6H), 4.34 (s, 2H), 6.76 – 7.59 (m, 11H) ppm; MS: m/z = 499 [M+] (20), 406 (61.5), 279 (100).
N-p-Tolyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoyl amide (3i). Red crystals (462 mg, 90%); m.p. 186-188 oC; IR: ν = 1664, 1594, 1564 cm-1; 1H-NMR: δ = 2.33 (s, 3H), 2.93 (s, 6H), 3.17 (s, 6H), 4.09 (s, 2H), 6.58 – 6.95 (m, 4H), 7.13 – 8.23 (m, 6H) ppm; MS: m/z = 513 [M+] (2), 406 (10), 387 (16), 279 (95), 107 (100).
N-m-Tolyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoyl amide (3j). Red crystals (446 mg, 87%); m.p. 155-157 oC; IR: ν = 1649, 1594, 1548 cm-1; 1H-NMR: δ = 2.35 (s, 3H), 2.92 (s, 6H), 2.98 (s, 6H), 4.08 (s, 2H), 6.66 – 6.96 (m, 4H), 7.07 – 7.47 (m, 6H) ppm; MS: m/z = 513 [M+] (18), 406 (65), 386 (24), 279 (100).
N-m-Ethoxyphenyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoyl amide (3k). Red crystals (445 mg, 82%); m.p. 120-122 oC; IR: ν = 1671, 1600, 1544 cm-1; 1H-NMR: δ = 1.33 (t, 3H), 2.90 (s, 6H), 3.10 (s, 6H), 4.06 (q, 2H), 4.18 (s, 2H), 6.59 – 7.29 (m, 6H), 7.51 – 8.23 (m, 4H) ppm; MS: m/z = 543 [M+] (8.8), 417 (5), 406 (65), 279 (100).
N-o-Chlorophenyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoyl amide (3l). Red crystals (427 mg, 80%); m.p. 124-125 oC; IR: ν = 1680, 1598, 1507 cm-1; 1H-NMR: δ = 2.89 (s, 6H), 2.99 (s, 6H), 4.15 (s, 2H), 6.56 – 7.55 (m, 8H), 7.96 (m, 1H), 8.55 (m, 1H) ppm; MS: m/z = 533 [M+] (15), 534 [M++1] (5), 406 (72), 280 (100), 279 (72 ).
N-Benzothiazol-2-yl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoyl amide (3m). Red crystals (378 mg, 68%); m.p. 108-109 oC; IR: ν = 1683, 1596, 1510 cm-1; 1H-NMR: δ = 2.90 (s, 6H), 3.05 (s, 6H), 4.28 (s, 2H), 6.53 – 7.33 (m, 6H), 7.45 – 8.40 (m, 4H) ppm. MS: m/z = 556 [M+] (16), 406 (44), 280 (77), 279 (95), 133 (100).
N-Methyl-N-phenyl 2-[4’-(dimethylamino)-2’-iodobenzyl]-4-dimethylaminobenzoyl amide (3n). Red crystals (333 mg, 65%); m.p. 97-99 oC; IR: ν = 1644, 1598, 1510 cm-1; 1H-NMR: δ = 2.91 (s, 6H), 2.96 (s, 3H), 3.18 (s, 6H), 4.00 (s, 2H), 6.69 – 7.28 (m, 7H), 7.46 – 7.49 (m, 2H), 8.13 – 8.23 (m, 2H) ppm; MS: m/z = 513 [M+] (2), 406 (62.5), 279 (100).