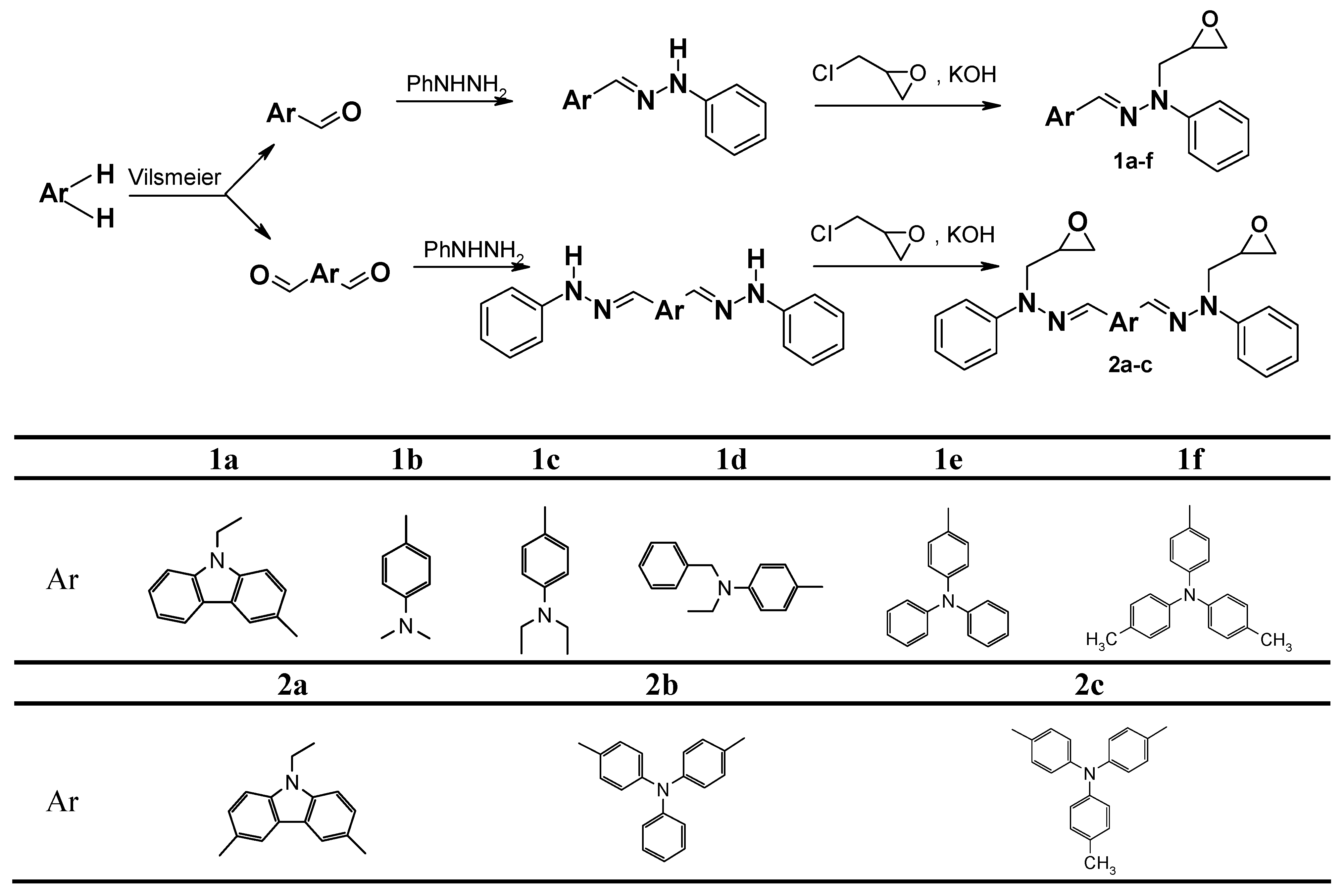
General method of the preparation of N-2,3-epoxypropylated phenylhydrazones 1a-f
To the mixture of phenylhydrazone of the corresponding aldehyde (1 mol) and epichlorohydrin (1.5˚mol), powdered 85 % potassium hydroxide (3 mol) and anhydrous Na2SO4 (0.4 mol) were added in three portions with prior cooling of the reaction mixture to 20-25˚°C (1st portion – 1/2 of Na2SO4 and 1/3 of KOH; 2nd portion – 1/4 of Na2SO4 and 1/3 of KOH after 1h from the beginning of the reaction; 3rd – 1/4 of Na2SO4 and 1/3 of KOH after 2h from the beginning of the reaction). The reaction mixture was stirred vigorously at 35-40˚°C until the starting hydrazone disappeared (3-4 h). After termination of the reaction, the mixture was cooled to RT and filtered off. The organic layer was washed with distilled water until the wash water was neutral. The organic layer was dried over anhydrous magnesium sulfate, treated with activated charcoal, filtered and excess of epiclorohydrin was removed. In the case of 1a-d the obtained residue was dissolved in a 1:1 mixture of toluene and 2-propanol. The crystals formed upon standing were filtered off and washed with 2-propanol. Compounds 1e,f were purified by column chromatography.
1-(9-ethylcarbazol-3-ylmethylene)-2-(2,3-epoxypropyl)-2-phenylhydrazine (1a): Yield 78.5 %, m.p. 136-137 οC (recrystallized from toluene); 1H-NMR spectrum (CDCl3, δ, 250 MHz): 8.35 (s, 1H, 4-HHt); 8.14 (d, J=7,8 Hz, 1H, 1-HHt); 7.93 (d, J=7,6 .Hz, 1H, 2-HHt); 7.90 (s, 1H, CH=N); 7.54-7.20 (m, 8H, Ph, Ht); 6.96 (t, J=7.2 Hz, 1H, 4-HPh); 4.37 (m, 3H, CH2CH3, one of the NCH2 protons); 4.04 (dd, J1=4.3 Hz, J2=16.4 Hz, 1H, next of the NCH2 protons); 3.32 (m, 1H, CH); 2.88 (dd, 1H, part of the ABX system, cis-HA of CH2O, JAX=2.6 Hz, JAB=4.9 Hz); 2.69 (dd, 1H, part of the ABX system, trans-HB of CH2O, JBX=4.0 Hz); 1.44 (t, J=7.2 Hz, 3H, CH3) ppm; Anal. Calcd. for C24H23N3O: C, 78.02; H, 6.27; N, 11.37. Found: C, 78.12; H, 6.18; N, 11.38.
2-(2,3-epoxypropyl)-1-(4-dimethylaminophenyl-methylene)-2-phenylhydrazine (1b): Yield 86.4 %; m.p. 123.5-124.5 οC (recrystallized from 2-propanol); 1H-NMR (CDCl3, δ, 80 MHz): 7.7-6.8 (m, 8H, CH=N, Ar); 6.7 (d, 2H, part of AB system, p-Ph); 4.5-3.7 (m, 2H, NCH2); 3.4-3.1 (m, 1H, CH); 2.9 (s, 6H, CH3); 2.9-2.7 (m, 1H, cis-H of CH2O); 2.7-2.5 (m, 1H, trans-H of CH2O) ppm; Anal. Calcd. for C18H21N3O: C, 73.19; H, 7.17; N, 14.23. Found: C, 73.15; H, 7.19; N, 14.31.
2-(2,3-epoxypropyl)-1-(4-diethylaminophenylmethylene)-2-phenylhydrazine (1c): Yield 80.4 %; m.p. 79-80.5 οC (recrystallized from ethyl ether); 1H-NMR (CDCl3, δ, 250 MHz): 7.7-6.7 (m, 8H, Ar, CH=N); 6.6 (d, 2H, 2-H, 6-H of p-Ph); 4.4-3.6 (m, 2H, NCH2CH); 3.6-3.0 (m, 5H, CH2CH3, CH2CHCH2); 2.75 (m, 1H, ABX, cis-HA of CH2O); 2.55 (m, ABX, trans-HB of CH2O); 1.1 (t, J=7.0 Hz, 6H, CH3) ppm; Anal. Calcd. for C20H25N3O: C, 74.27; H, 7.79; N, 12.99. Found: C, 74.21; H, 7.70; N, 12.91.
1-(4-benzylethylaminophenylmethylene)-2-(2,3-epoxypropyl)-2-phenylhydrazine (1d): Yield of oily 1d 80.4 %; 1H-NMR (CDCl3, δ, 400 MHz): 7.70 (s, 1H, CH=N); 7.55 (d, 2H, J=8.8 Hz, 2,6-Hp-Ph); 7.16-7.41 (m, 4H, Ar); 6.93 (t, 1H, J=7.3 Hz, 4-HPh); 6.69 (d, 2H, J=8.8 Hz, 3,5-Hp-Ph); 4.58 (s, 2H, CH2Ph); 4.37 (dd, 1H, ABX, JAB=16.2, JAX=2.4 Hz, HA of NCH2); 3.99 (dd, 1H, ABX, JBX=4.1 Hz, HB of NCH2); 3.28 (m, 1H, CHX); 3.53 (q, 2H, J=7.3 Hz, CH2CH3); 2.84 (dd, 1H, ABX, JAB=4.8 Hz, JAX=2.7 Hz, cis-HA of CH2O); 2.62 (dd, 1H, ABX, JBX=4.0 Hz, trans-HB of CH2O); 1.24 (t, J=7.3 Hz, 3H, CH2CH3); Anal. Calcd. for C25H27N3O: C, 77.89; H, 7.06; N, 10.90. Found: C, 77.91; H, 7.15; N, 10.81.
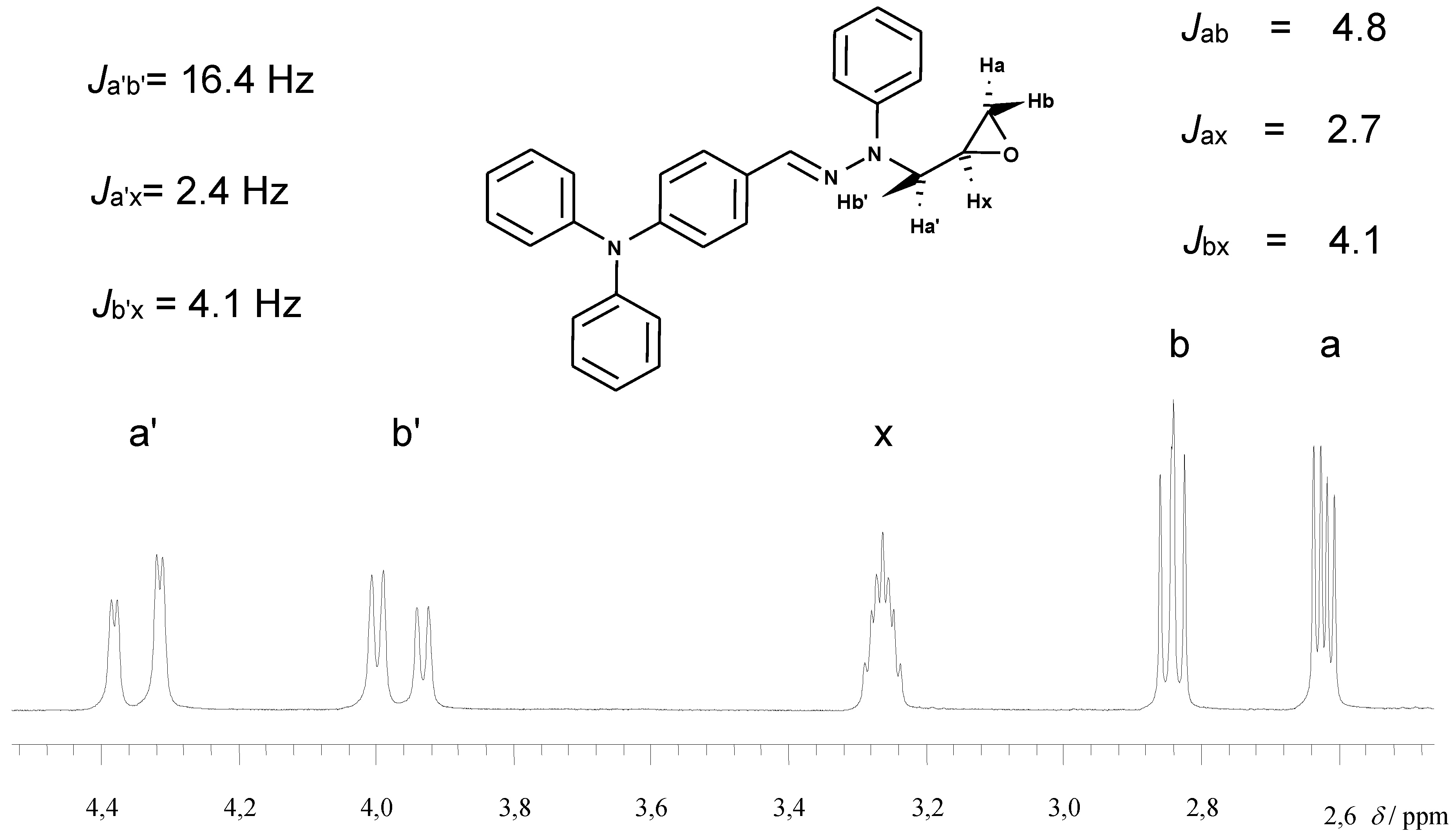
1-[4-(diphenylamino)phenylmethylene]-2-(2,3-epoxypropyl)-2-phenylhydrazine (1e): Yield 89.9 %; m.p. 141-142.5 οC (recrystallized from toluene); 1H-NMR (CDCl3, δ, 250 MHz 250): 7.65-6.98 (m, 19H, CH=N, Ar); 6.93 (t, J=7.2 Hz, 1H, 4-HPh); 4.35 (dd, 1H, part of the ABX system, HA of NCH2, JAX=2.4 Hz, JAB=16.4); 3.99 (dd, 1H, part of the ABX system, HB of NCH2, JBX=4.1 Hz); 3.26 (m, 1H, CH); 2.84 (dd, 1H, part of the ABX system, cis- HA of CH2O, JAX=2.7 Hz, JAB=4.8 Hz); 2.62 (dd, 1H, part of the ABX system, trans-HB of CH2O, JBX=4.1 Hz); Anal. Calcd. for C28H25N3O: C, 80.16; H, 6.01; N, 10.02. Found: C, 80.19; H, 6.10; N, 10.09.
2-(2,3-epoxypropyl)-1-[4-(4,4ۥ-dimethyldiphenylaminophenylmethylene]-2-phenylhydrazine (1f): Yield of oily 1f 87.3 %; 1H-NMR (CDCl3, δ, 250 MHz): 7.62 (s, 1H, CH=N); 7.55-6.90 (m, 17H, Ar); 4.34 (dd, 1H, part of the ABX system, HA of NCH2, JAX=2.2 Hz, JAB=16.5); 3.98 (dd, 1H, part of the ABX system, HB of NCH2, JBX=4.4 Hz); 3.27 (m, 1H, CH); 2.85 (dd, 1H, part of the ABX system, cis- HA of CH2O, JAX=2.7 Hz, JAB=4.9 Hz); 2.63 (dd, 1H, part of the ABX system, trans-HB of CH2O, JBX=4.0 Hz). Ph); 7.72 (s, 1H, CH=N); Anal. Calcd. for C30H29N3O: C, 80.51; H, 6.53; N, 9.39. Found: C, 80.52; H, 6.48; N, 9.46.
General synthetic method of bis(N-2,3-epoxypropyl-N-phenyl)hydrazones 2a-c
To the mixture of the diphenylhydrazone of the corresponding dialdehyde (1 mol) and epichlorohydrin (22.5˚mol), powdered 85 % potassium hydroxide (4.5 mol) and anhydrous Na2SO4 (0.6 mol) were added in three portions with prior cooling of the reaction mixture to 20-25˚°C (1st portion – 1/2 of Na2SO4 and 1/3 of KOH; 2nd portion – 1/4 of Na2SO4 and 1/3 of KOH after 1h from the beginning of the reaction; 3rd – 1/4 of Na2SO4 and 1/3 of KOH after 2h from the beginning of the reaction). The reaction mixture was stirred vigorously at 35-40˚°C until the starting dihydrazone disappeared (7-8 h). After completion of the reaction, the mixture was cooled to RT and filtered off. The organic part was washed with distilled water until the wash water was neutral. The organic layer was dried over anhydrous magnesium sulfate, treated with activated charcoal, filtered and the excess of epiclorohydrin was removed. In the case of 2a,b the residues obtained were recrystallized from toluene and the crystals formed upon standing were filtered off and washed with 2-propanol. Compound 2c was purified by column chromatography.
9-ethyl-3,6-carbazoledicarbaldehyde bis(N-2,3-epoxypropyl-N-phenyl)hydrazone (2a): Yield: 342 g (63 %), m.p. 119-120 οC; 1H-NMR spectrum (300 MHz, CDCl3), ppm: 8.38 (split s, 2H); 7.9-7.88 (m, 4H); 7.49-7.43 (m, 4H); 7.40-7.32 (m, 6H); 6.96 (t, 2H, J= 7.2 Hz); 4.42-4.29 (m, 6H); 4.06-3.97 (dd, 2H, (HA), JAX= 4.5 Hz, JAB= 16.4 Hz); 3.31 (m, 2H); 2.90-2.85 (dd, 2H, (HA), JAX= 3.9 Hz); 2.70-2.65 (dd, 2H, (HB), JBX= 2.7 Hz; JAB=5.1 Hz); 1.43 (t, J=7.2 Hz); Anal. Calcd for C34H33N5O2%: C 75.11; H 6.12; N 12.88. Found, %: C 75.16; H 6.09; N 12.81.
4-(4-formyldiphenylamino)benzaldehyde bis(N-2,3-epoxypropyl-N-phenyl)hydrazone (2b): Yield: 312 g (52 %), m.p. 163.5-165 οC; 1H-NMR spectrum (300 MHz, CDCl3), ppm: 7.63 (s, 2H); 7.62-7.56 (m, 4H); 7.43-7.02 (m, 17H); 6.94 (t, 2H, J= 7.1 Hz); 4.40-4.30 (dd, 2H, (HA), JAX= 2.1 Hz, JAB= 16.5 Hz); 4.02-3.92 (dd, 2H, (HB), JBX= 4.2 Hz); 3.26 (m, 2H); 2.84 (dd, 2H, (HA), JAX= 4.2 Hz, JAB= 5.1 Hz); 2.65-2.60 (dd, (HB), JBX= 2.7 Hz); Anal. Calcd for C38H35N5O2%: C 76.87; H 5.94; N 11.80. Found, %: C 76.71; H 5.91; N 11.70.
4-(4-formyl-4ۥ-methyldiphenylamino)benzaldehyde bis(N-2,3-epoxypropyl-N-phenyl)hydrazone (2c): Yield of amorphous 2c: 332 g (55 %); 1H-NMR spectrum (300 MHz, CDCl3), ppm: 7.63 (s, 2H); 7.61-7.54 (m, 4H); 7.42-7.02 (m, 17H); 6.94 (t, 2H, J= 7.2 Hz); 4.40-4.28 (dd, 2H, (HA), JAX= 2.1 Hz, JAB= 16.5 Hz); 4.02-3.90 (dd, 2H, (HB), JBX= 4.2 Hz); 3.26 (m, 2H); 2.84 (dd, 2H, (HA), JAX= 4.2 Hz, JAB= 4.8 Hz); 2.66-2.60 (dd, (HB), JBX= 2.7 Hz) ; 2.33 (s, 3H); Anal. Calcd for C39H37N5O2%: C 77.08; H 6.14; N 11.52. Found, %: C 77.14; H 6.10; N 11.58.