Ionic Liquid-promoted Ring-closure Reactions between 1,4-Dihydroxyanthraquinone and Diamines
Abstract
:Introduction
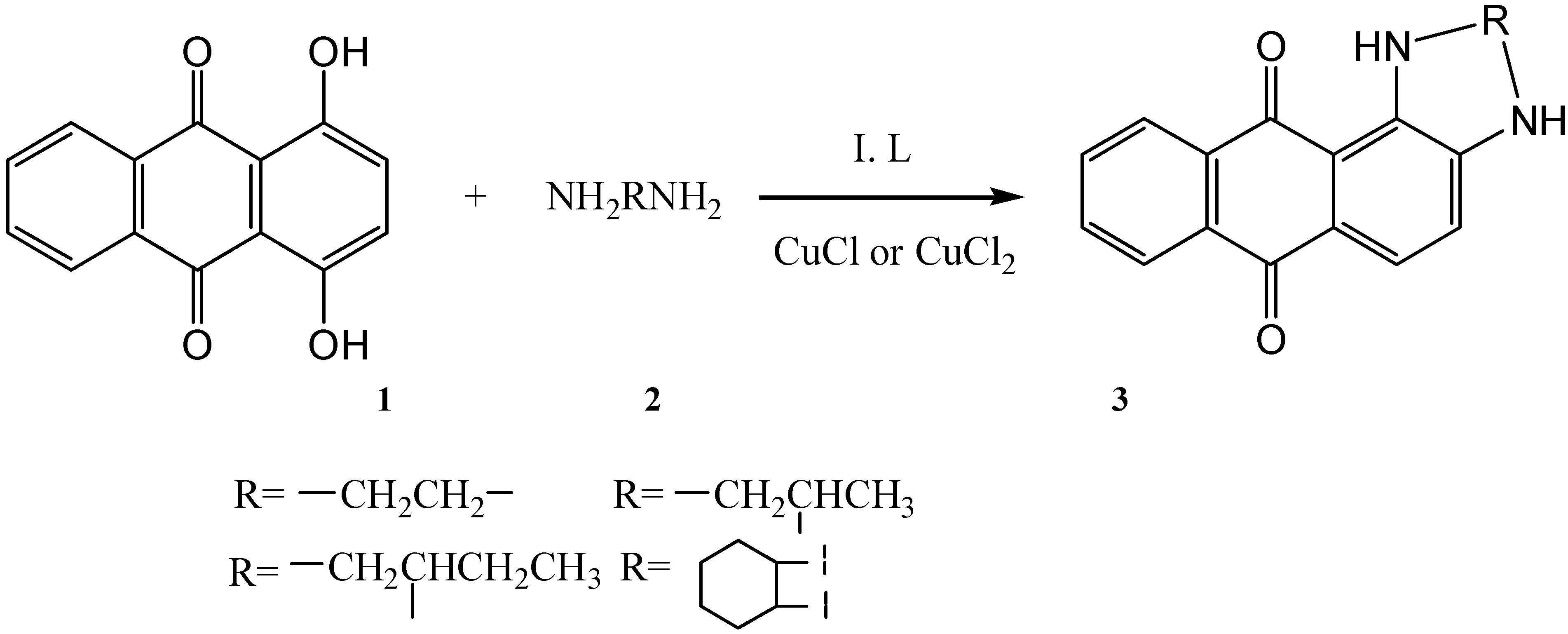
Results and Discussion
| Entry | Diamine | Solvent and Copper salt | Reaction time (h) | Yield b (%) |
| 1 | ethylenediamine | [Bmim]PF6+ CuCl2 | 2 | 99 |
| 2 | ethylenediamine | [Bmim]PF6+ CuCl | 2 | 95 |
| 3 | ethylenediamine | [Bmim]Cl·CuCl | 2 | 87 |
| 4 | ethylenediamine | [Bmim]BF4+ CuCl | 2 | 90 |
| 5 | ethylenediamine | [Bmim]BF4+ CuCl2 | 2 | 96 |
| 6 | ethylenediamine | CuCl2 + DMF | 8 | 56 |
| 7 | ethylenediamine | CuCl + DMF | 8 | 42 |
| 8 | ethylenediamine | CuCl2 + CH2Cl2 | 8 | 50 |
| 9 | ethylenediamine | CuCl + CH2Cl2 | 8 | 40 |
| 10 | 1,2-propanediamine | [Bmim]PF6+ CuCl2 | 2 | 92 |
| 11 | 1,2-cyclohexanediamine | [Bmim]PF6+ CuCl2 | 2 | 80 |
| 12 | N-methylenthylenediamine | [Bmim]PF6+ CuCl2 | 2 | 48 |
| 13 | N-ethylenthylenediamine | [Bmim]PF6+ CuCl2 | 2 | 31 |
Conclusions
Experimental Section
General
General synthetic procedure
Acknowledgements
References
- Patrice, J. G.; John, M. M.; Glynn, D. M. Reaction of Imines of Aminoanthraquinones with Formaldehyde and Alkenes. Tetrahedron 1995, 51, 6133–6144. [Google Scholar] [CrossRef]
- Krapcho, A. P.; Petry, M. E.; Getahun, Z.; Landi, J. J.; Stallman, J.; Polsenberg, J. F.; Gallagher, C. E.; Maresch, M.J.; Hacker, M. P.; Giliani, F.C.; Beggiolin, G.; Pezzoni, G.; Menta, E.; Manzotti, C.; Oliva, A.; Spinelli, S.; Tognella, S. 6,9-Bis[(aminoalkyl)amino]benzo[g]-isoquinoline-5,10-diones. A Novel Class of Chromophore-Modified Antitumor Anthracene-9,10-diones: Synthesis and Antitumor Evaluations. J. Med. Chem. 1994, 37, 828–837. [Google Scholar]
- Amitage, B.; Yu, C.; Devadoss, C.; Schuster, G. B. Cationic Anthraquinone Derivatives as Catalytic DNA Photonucleases: Mechanisms for DNA Damage and Quinone Recycling. J. Am. Chem. Soc. 1994, 116, 9847–9859. [Google Scholar] [CrossRef]
- Magnus, P.; Eisenbeis, S. A.; Magnus, N. A. A Concise Sythesis of the Anthraquinone Portion of Dynemicin-A. J. Chem. Soc. Chem. Comm. 1994, 1545–1546. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Gross, J. L.; Kerr, M. A.; Lemus, R. H.; Ikeda, K.; Ohe, K. Synthesis of the Anthraquinone Framework of Dynemicin A. Angew. Chem. Int. Ed. Engl. 1994, 33, 781–783. [Google Scholar] [CrossRef]
- Toshio, T.; Masaru, M.; Teijiro, K. A Novel Ring-closure Reaction between 1,4-Dihydroxy-anthraquinone and Diamines Promoted by Copper Ions. Bull. Chem. Soc. Jpn. 1981, 54, 2735–2738. [Google Scholar] [CrossRef] Toshio, T.; Masaru, M.; Teijiro, K. A Novel Ring Closure Reaction between 1,4-Dihydroxyanthraquinone and Diamines Promoted by Copper Ions. Chem. Lett. 1980, 743–744. [Google Scholar]
- Le, Z. G.; Chen, Z. C.; Hu, Y.; Zheng, Q. G. Organic Reaction in Ionic Liquids: N-alkylation of Phthalimide and Several Nitrogen Heterocycles. Synthesis 2004, 208–212. [Google Scholar] Le, Z. G.; Chen, Z. C.; Hu, Y.; Zheng, Q. G. Organic Reactions in Ionic liquids: ionic liquid-promoted efficient synthesis of N-alkyl and N-arylimides. Synthesis 2004, 995–998. [Google Scholar] Le, Z. G.; Chen, Z. C.; Hu, Y.; Zheng, Q. G. Organic reactions in ionic liquids: A Simple and Highly Regioselective N-Substitution of Pyrrole. Synthesis 2004, 1951–1954. [Google Scholar] Le, Z. G.; Chen, Z. C.; Hu, Y.; Zheng, Q. G. Organic reactions in ionic liquids: an efficient method for the N-alkylation of benzotriazoles. J. Chem. Res. (S) 2004, 344–346. [Google Scholar] [CrossRef] Le, Z. G.; Chen, Z. C.; Hu, Y.; Zheng, Q. G. Organic Reactions in Ionic liquids: Organic Reactions in Ionic liquids: a simple highly regioselective or regiospecific substitutions of benzotriazole. Heterocycles 2004, 63, 1077–1081. [Google Scholar] [CrossRef]
- Wassercheid, P.; Welton, T. (Eds.) Ionic liquids in Synthesis; Wiley-VCH: Weinheim, Germany, 2002.
- Sample availability: Contact the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Le, Z.-G.; Xie, Z.-B.; Ying, M. Ionic Liquid-promoted Ring-closure Reactions between 1,4-Dihydroxyanthraquinone and Diamines. Molecules 2006, 11, 464-468. https://doi.org/10.3390/11060464
Le Z-G, Xie Z-B, Ying M. Ionic Liquid-promoted Ring-closure Reactions between 1,4-Dihydroxyanthraquinone and Diamines. Molecules. 2006; 11(6):464-468. https://doi.org/10.3390/11060464
Chicago/Turabian StyleLe, Zhang-Gao, Zong-Bo Xie, and Min Ying. 2006. "Ionic Liquid-promoted Ring-closure Reactions between 1,4-Dihydroxyanthraquinone and Diamines" Molecules 11, no. 6: 464-468. https://doi.org/10.3390/11060464
APA StyleLe, Z.-G., Xie, Z.-B., & Ying, M. (2006). Ionic Liquid-promoted Ring-closure Reactions between 1,4-Dihydroxyanthraquinone and Diamines. Molecules, 11(6), 464-468. https://doi.org/10.3390/11060464




