Synthesis and Molecular Structures of Two [1,4-bis(3-pyridyl)-2,3-diazo-1,3-butadiene]-dichloro-Zn(II) Coordination Polymers
Abstract
:Introduction
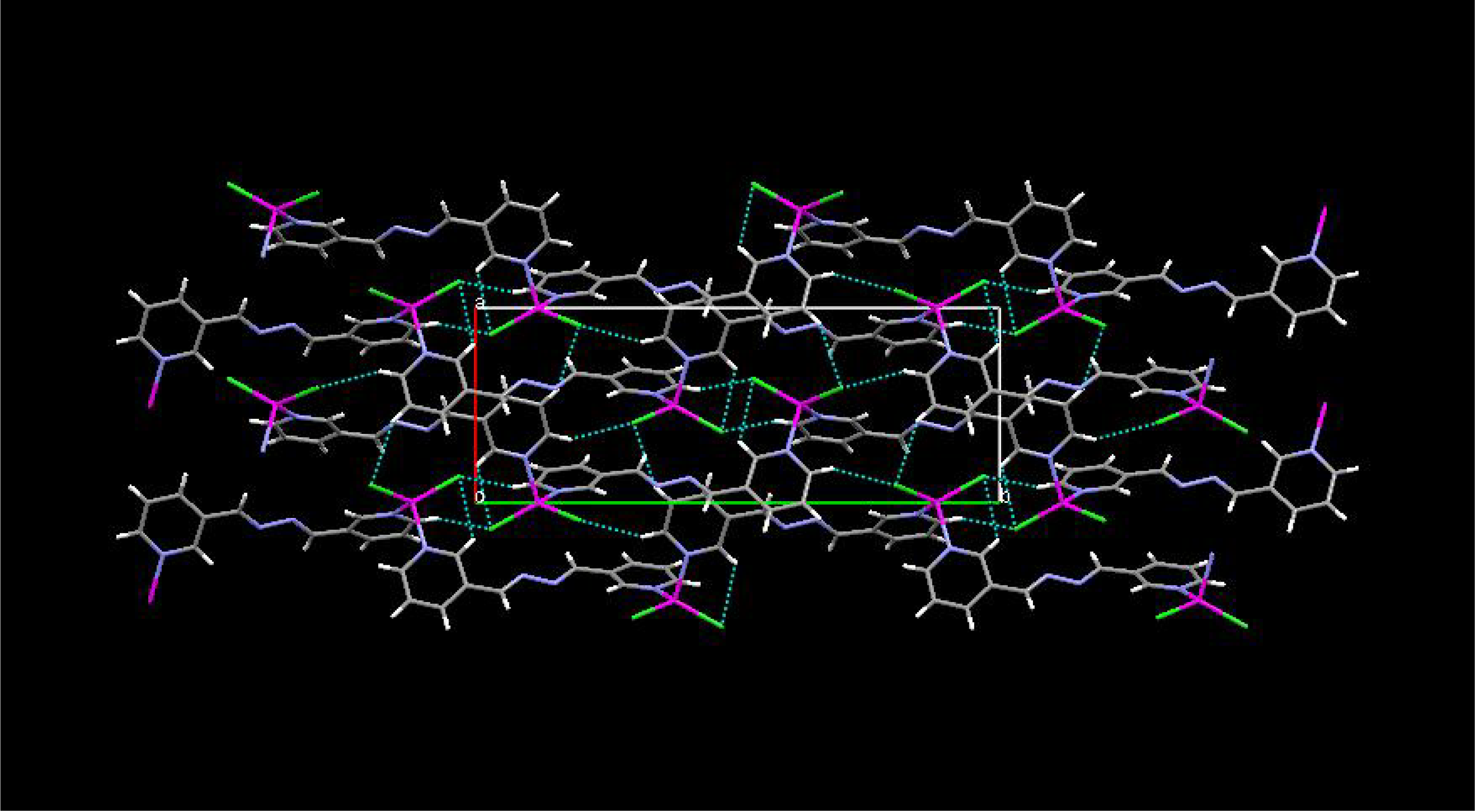
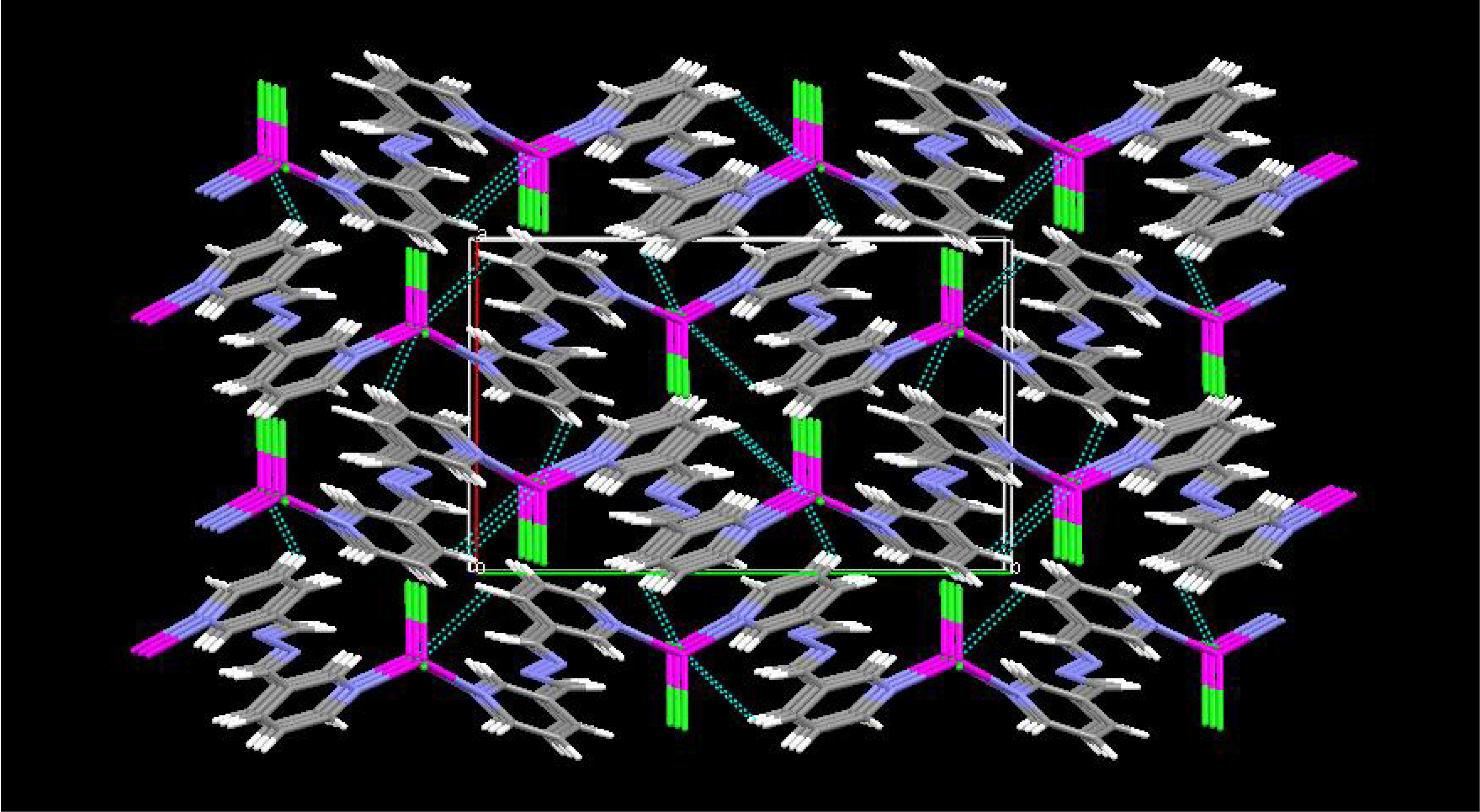
Results and Discussion
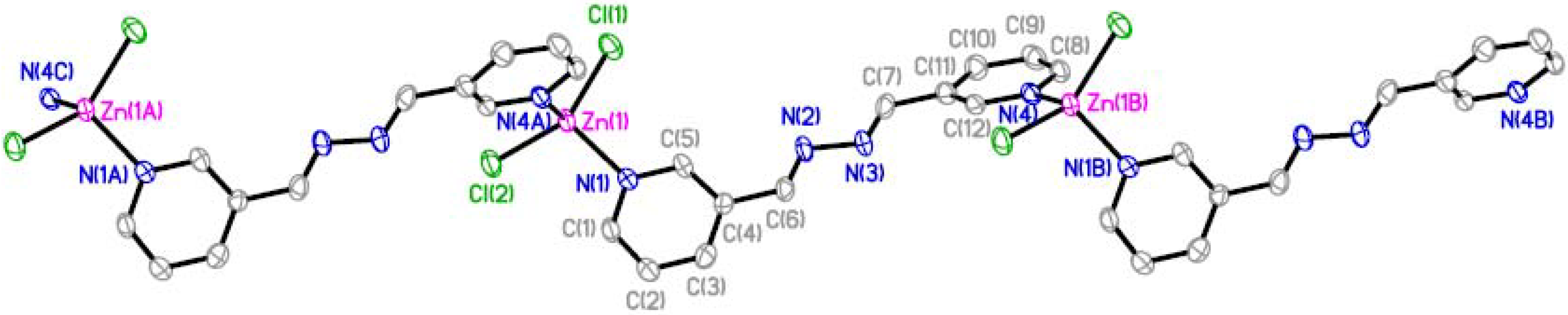
| Bond Length [Å] | Bond Angle [°] | ||
|---|---|---|---|
| Zn(1)-Cl(1) | 2.2229(11) | N(1)-Zn(1)-N(4A) | 99.56(13) |
| Zn(1)-Cl(2) | 2.2123(9) | N(1)-Zn(1)-Cl(2) | 110.25(10) |
| Zn(1)-N(1) | 2.049(3) | N(4A)-Zn(1)-Cl(2) | 104.18(9) |
| Zn(1)-N(4A) | 2.057(3) | N(1)-Zn(1)-Cl(1) | 107.14(9) |
| N(2)-C(6) | 1.281(5) | N(4A)-Zn(1)-Cl(1) | 107.54(9) |
| N(2)-N(3) | 1.415(4) | Cl(2)-Zn(1)-Cl(1) | 125.13(4) |
| N(3)-C(7) | 1.272(5) | C(6)-N(2)-N(3) | 109.6(3) |
| C(4)-C(6) | 1.477(5) | C(7)-N(3)-N(2) | 112.1(3) |
| C(7)-C(11) | 1.467(5) | N(2)-C(6)-C(4) | 120.0(4) |
| N(3)-C(7)-C(11) | 119.5(4) | ||

| Bond Length [Å] | Bond Angle [°] | ||
|---|---|---|---|
| Zn(1)-Cl(1) | 2.2205(6) | N(1)-Zn(1)-N(4A) | 116.22(8) |
| Zn(1)-Cl(2) | 2.2336(7) | N(1)-Zn(1)-Cl(2) | 104.83(6) |
| Zn(1)-N(1) | 2.047(2) | N(4A)-Zn(1)-Cl(2) | 107.56(6) |
| Zn(1)-N(4A) | 2.052(2) | N(1)-Zn(1)-Cl(1) | 103.00(6) |
| N(2)-C(6) | 1.262(3) | N(4A)-Zn(1)-Cl(1) | 105.31(6) |
| N(2)-N(3) | 1.415(3) | Cl(2)-Zn(1)-Cl(1) | 120.49(3) |
| N(3)-C(7) | 1.274(3) | C(6)-N(2)-N(3) | 111.7(2) |
| C(4)-C(6) | 1.473(3) | C(7)-N(3)-N(2) | 111.5(2) |
| C(7)-C(11) | 1.466(3) | N(2)-C(6)-C(4) | 121.3(2) |
| N(3)-C(7)-C(11) | 120.7(2) | ||
Conclusions
Experimental
General
Synthesis of compound 1
Synthesis of compound 2
X-ray techniques
| Compound | 1 | 2 |
|---|---|---|
| Formula | C12H10N4Cl2Zn | C12H10N4Cl2Zn |
| Formula weight | 346.51 | 346.51 |
| Crystal system | Orthorhombic | monoclinic |
| Space group | Pna21 | P21/n |
| Formula per unit cell, Z | 4 | 4 |
| Unit-cell dimensions | a = 7.9652(3) Å | a = 9.1752(3) Å |
| b = 21.4716(7) Å | b = 14.5976(4) Å | |
| c = 8.2491(3) Å | c = 10.3666(3) Å | |
| α = β = γ = 90° | α = γ = 90°, β = 98.231(2)° | |
| Unit-cell volume, V (Å 3) | 1410.81(9) | 1374.16(7) |
| Dcalcd. (g/cm3) | 1.631 | 1.675 |
| Absorption coefficient, μ(mm-1) | 2.109 | 2.165 |
| F(000) | 696 | 696 |
| Crystal size (mm) | 0.50 x 0.08 x 0.05 | 0.25 x 0.15 x 0.03 |
| θ ranges (o) for data collection | 1.90 ~ 27.50 | 2.43 ~ 27.50 |
| Index ranges | -10 ≦ h ≦ 10 | -11 ≦ h ≦ 11 |
| -27 ≦ k ≦ 23 | -17 ≦ k ≦ 18 | |
| -10 ≦ l ≦ 10 | -13 ≦ l ≦ 13 | |
| Reflections collected | 9123 | 9083 |
| Independent reflections | 3184 (Rint = 0.0641) | 3145 (Rint = 0.0453) |
| Completeness to θ=27.50 (%) | 100.0 | 99.7 |
| Absorption correction | Multi-scan | Multi-scan |
| Max. and min. transmission | 0.900 and 0.418 | 0.939 and 0.700 |
| Refinement method | Full-matrix L. S. on F2 | Full-matrix L. S. on F2 |
| Data / restraints / parameters | 3184 / 1 / 172 | 3145 / 0 / 173 |
| Goodness-of-fir on F2 | 1.007 | 1.013 |
| Final R indices [I > 2σ(I)] | R1 = 0.0398, wR2 = 0.0695 | R1 = 0.0326, wR2 = 0.0598 |
| R indices (all data) | R1 = 0.0718, wR2 = 0.0765 | R1 = 0.0694, wR2 = 0.0672 |
| Largest diff. peak and hole (e/ Å 3) | 0.320 and -0.394 | 0.347 and -0.363 |
Acknowledgements
References
- Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2003, 2781–2804. [Google Scholar] [CrossRef]
- Barnett, S. A.; Champness, N. R. Structural diversity of building-blocks in coordination Frame-work synthesis—combining M(NO3)2 junctions and bipyridyl ligands. Coord. Chem. Rev. 2003, 246, 145–168. [Google Scholar] [CrossRef]
- Hagrman, P. J.; Hagrman, D.; Zubieta, J. Organic-Inorganic Hybrid Materials: From “Simple” Coordination Polymers to Organodiamine-Templated Molybdenum Oxides. Angew. Chem. Int. Ed. Engl. 1999, 38, 2638–2684. [Google Scholar] [CrossRef]
- Prior, T. J.; Bradshaw, D.; Teat, S. J.; Rosseinsky, M. J. Designed layer assembly: a three-dimensional framework with 74% extra-framework volume by connection of infinite two-dimensional sheets. Chem. Commun. 2003, 500–501. [Google Scholar]
- Tseng, B. C.; Chen, B. S.; Lee, S. Y.; Liu, W. H.; Lee, G. H.; Peng, S. M. Anion-directed assembly of supramolecular zinc(II) halides with N,N'-bis-4-methyl-pyridyl oxalamide. New J. Chem. 2005, 29, 1254–1257. [Google Scholar]
- Dong, Y. B.; Smith, M. D.; zur Loye, H. C. New Inorganic–Organic Coordination Polymers Generated from Rigid or Flexible Bidentate Ligands and Co(NCS)2·xH2O. J. Solid State Chem. 2000, 155, 143–153. [Google Scholar] [CrossRef]
- Dong, Y. B.; Smith, M. D.; Layland, R. C.; zur Loye, H. C. A Novel Noninterpenetrating Polycyclohexane Network: A New Inorganic/Organic Coordination Polymer Structural Motif Generated by Self-Assembly of "T-Shaped" Moieties. Chem. Mater. 2000, 12, 1156–1161. [Google Scholar] [CrossRef]
- Yaghi, O. M.; Li, Hailian; Groy, T. L. Construction of Porous Solids from Hydrogen-Bonded Metal Complexes of 1,3,5-Benzenetricarboxylic Acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Blake, A. J.; Hill, S. J.; Hubberstey, P.; Li, W. S. Rectangular grid two-dimensional sheets of copper(II) bridged by both co-ordinated and hydrogen bonded 4,4´-bipyridine (4,4´-bipy) in [Cu(μ-4,4´-bipy)(H2O)2(FBF3)2]·4,4´-bipy. J. Chem. Soc., Dalton Trans. 1997, 913–914. [Google Scholar]
- Yang, C. H.; Chuo, C. M.; Lee, G. H.; Wang, C. C. Self-assembly of two mixed-ligands metal-organic coordination polymers, [MII2(DPA)2(C4O4)(C2O4)] (M = Cu, Zn). Inorg. Chem. Comm. 2003, 6, 135–140. [Google Scholar] [CrossRef]
- COLLECT. Nonius 2000, Nonius BV: Delft, The Netherlands, 2000.
- Otwinowski, Z.; Minor, W. Method. Enzymol. 1997, 276, 307–326. [CrossRef]
- Blessing, R. H. An empirical correction for absorption anisotropy. Acta Cryst., Sect. A. 1995, 51, 33–38. [Google Scholar] [CrossRef]
- Sheldrick, G. M. Phase annealing in SHELX-90: direct methods for larger structures. Acta Cryst. 1990, A46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G. M. SHELXL97, Program for the refinement of crystal structure, University of Göttingen: Göttingen, Germany, 1997.
- SHELXTL V6.1, Bruker AXS, Inc: Madison, Wisconsin, USA, 2000.
- Bruno, I. J.; Cole, J. C.; Edgington, P. R.; Kessler, M.; Macrae, C. F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Cryst. 2002, B58, 389–397. [Google Scholar]
- Sample Availability: Samples of the title compounds may be obtained from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Lee, G.-H.; Wang, H.-T. Synthesis and Molecular Structures of Two [1,4-bis(3-pyridyl)-2,3-diazo-1,3-butadiene]-dichloro-Zn(II) Coordination Polymers. Molecules 2006, 11, 589-596. https://doi.org/10.3390/11080589
Lee G-H, Wang H-T. Synthesis and Molecular Structures of Two [1,4-bis(3-pyridyl)-2,3-diazo-1,3-butadiene]-dichloro-Zn(II) Coordination Polymers. Molecules. 2006; 11(8):589-596. https://doi.org/10.3390/11080589
Chicago/Turabian StyleLee, Gene-Hsiang, and Hsin-Ta Wang. 2006. "Synthesis and Molecular Structures of Two [1,4-bis(3-pyridyl)-2,3-diazo-1,3-butadiene]-dichloro-Zn(II) Coordination Polymers" Molecules 11, no. 8: 589-596. https://doi.org/10.3390/11080589




