Abstract
The synthesis of optically active [N-tosyl-(R)-prolyloxy]-2(S)-[4-cyano-8,8-ethylenedioxy-5-oxo-5,6,7,8-tetrahydroindolizin-3-yl] acetate (4a), a key intermediate for the total asymmetric synthesis of 20(S)-camptothecin anticancer drugs, is described. Its structure was characterized by 2D-NMR techniques and the absolute configuration was further confirmed for the first time by X-ray crystal structure analysis.
Introduction
20(S)-Camptothecins [20(S)-CPTs] (Figure 1) have attracted lasting interest in organic synthetic chemistry due to their unique chemical structures and potent antitumor activities, and considerable effort has been devoted over the last thirty years to the development of elegant methods for the total synthesis of 20(S)-CPTs [1]. Among the numerous asymmetric synthesis approaches to these alkaloids described, the strategy utilizing the (S)-configuration tricyclic hydroxylactone 2 as a key chiral intermediate promises the most efficient industrial scale synthesis to 20(S)-CPTs. To date three synthetic methods for the preparation of compound 2 have been reported using a chiral auxiliary to induce asymmetry [2], a chemocatalyst to catalyze asymmetric synthesis [3] and optical resolution [4]. We were especially interested in the asymmetric synthesis of compound 2 developed by Tagawa et al. [2] for its use of commercially available starting materials, mild reaction conditions and simple manipulations.
As a part of our continuing program [5] to explore a practical and commercial route of 20(S)-CPTs, herein we report the preparation and structural analysis of [N-tosyl-(R)-prolyloxy]-2(S)-[4-cyano-8,8-ethylenedioxy-5-oxo-5,6,7,8-tetrahydroindolizin-3-yl] acetate (4a), which is the key intermediate in the synthesis of lactone 2.
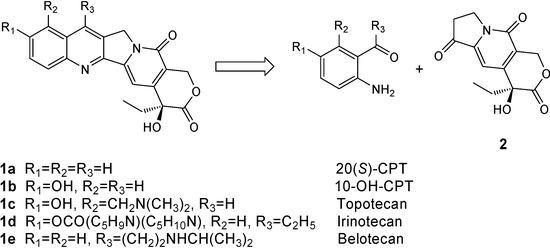
Figure 1.
Structures of 20(S)-camptothecin and its clinic drugs.
Results and Discussion
The synthesis of optically active hydroxyl lactone 2 is depicted in Scheme 1. Firstly, 2-bromo- indolizine analogue 3, prepared by the reported methods [6], was condensed with N-tosyl-(R)-proline to give a diastereoisomeric mixture 4a and 4b. Subsequently, the mixture afforded the appropriate optical active compound 5a under the asymmetric induction of the chiral auxiliary reagent N-tosyl-(R)-proline via the diastereoselective ethylation and fractional crystallization from isopropanol. Finally, compound 5a was treated by similar processes [2] to produce the key intermediate tricyclic hydroxyl lactone 2.
Diastereoisomers 4a and 4b were obtained as a low melting (49.6-61°C) 1:1 mixture inseparable by chromatographic purification. However, it is interesting to note that the crude 4a/4b mixture could be converted into isomer 4a with a high melting point of 172.6-174.3˚C in 70% yield by crystallization from methanol. This result was evident from the changes in their respective 1H-NMR spectra. In the spectrum of the mixture there were two sets of signals (δH 6.65, 6.63 and δH 6.30, 6.19), representing the H-C(10) and H-C(2) protons of the two diastereoisomers 4a and 4b, respectively. After crystallization, the 1H-NMR spectrum only showed the δH 6.65 and 6.30 signals of one isomer. The complete proton and carbon chemical shift assignment for the structure of isomer 4a was established by the combination of 1D and 2D NMR experiments such as DEPT, H-H COSY, as well as HMQC and HMBC (see Table 1 and Figure 2). By comparing 1H-NMR spectra data with those of similar compounds [4c], we elucidated the new chiral carbon atom C(2) to be (S)-configuration.
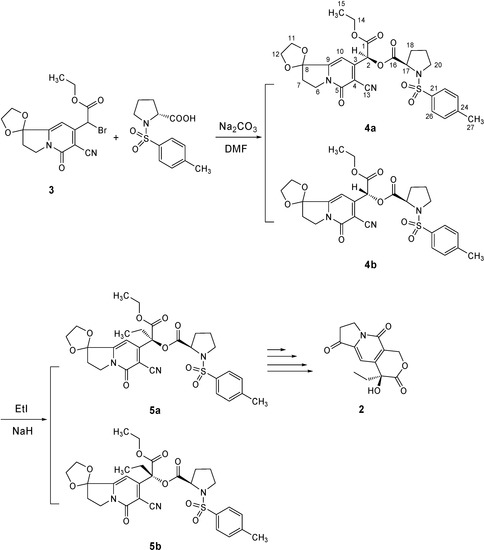
Scheme 1.
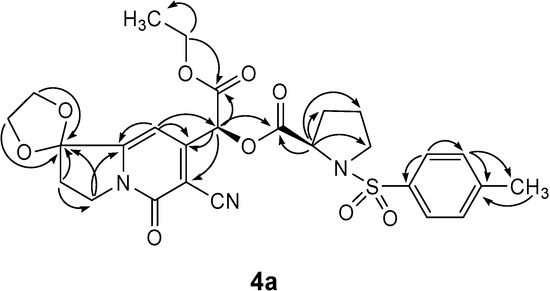
Figure 2.
Important HMBC (→) correlations of compound 4a.

Table 1.
1H- and 13C-NMR data for 4a in CDCl3; δ in ppm, J in Hz.
| Position | δH | δC |
|---|---|---|
| 1 | 165.2 (s) | |
| 2 | 6.30 (s) | 71.9 (d) |
| 3 | 103.7 (s) | |
| 4 | 154.1 (s) | |
| 5 | 158.3 (s) | |
| 6 | 4.22 (m, Ha), 4.11 (m, Hb) | 45.7 (t) |
| 7 | 2.44 (m) | 33.5 (t) |
| 8 | 112.9 (s) | |
| 9 | 154.4 (s) | |
| 10 | 6.65 (s) | 97.9 (s) |
| 11 | 4.31 (m, Ha), 4.11 (m, Hb) | 65.7 (t) |
| 12 | 4.31 (m, Ha), 4.11 (m, Hb) | 66.3 (t) |
| 13 | 113.7 (s) | |
| 14 | 4.31 (m, Ha), 4.22 (m, Hb) | 63.1 (t) |
| 15 | 1.30 (t, J = 7.1) | 13.9 (q) |
| 16 | 170.1 (s) | |
| 17 | 4.40 (dd, J = 3.1, 5.6) | 60.6 (d) |
| 18 | 2.20 (m, Ha), 1.97 (m, Hb) | 30.9 (t) |
| 19 | 2.10 (m, Ha), 1.80 (m, Hb) | 24.5 (t) |
| 20 | 3.27 (m, Ha), 3.60 (m, Hb) | 48.6 (t) |
| 21 | 143.9 (s) | |
| 22 | 7.72 (d, J = 8.2) | 127.3 (d) |
| 23 | 7.31 (d, J = 8.0) | 129.8 (d) |
| 24 | 134.7 (s) | |
| 25 | 7.31 (d, J = 8.0) | 129.8 (d) |
| 26 | 7.72 (d, J = 8.2) | 127.3 (d) |
| 27 | 2.43 (s) | 21.5 (q) |
To further confirm the absolute configuration of isomer 4a, we performed an X-ray crystal diffraction analysis. A perspective view and the numbering scheme adopted for the 4a molecule is depicted in Figure 3. The known (R)-configuration of the chiral atom C(17) of compound 4a was characterized by the chemical correlation, and the O(6)-C(16)-C(17)-N(3) torsion angle of -163.7(3)º and the O(6)-C(16)-C(17)-C(18) torsion angle of 82.8(4)º. As a result, the new stereogenic carbon C(2) is confirmed to be (S)-configuration by the C(3)-C(2)-O(6)-C(16) torsion angle of 171.8(3)º and the C(1)-C(2)-O(6)-C(16) torsion angle of -69.4(5)º. The pyrrolidine ring of N-tosyl-(R)-proline segment is in an envelope conformation with the flap atom C18 lying 0.553(7)Å from the C(17)/N(3)/C(20)/C(19) plane. The phenyl ring almost locates at the right-angled position of the C(17)/N(3)/C(20)/C(19) plane with a dihedral angle of 79.0(2)º. Another pyrrolidine ring fused with pyridone ring is also in an envelope conformation with the flap atom C(7) lying 0.442(8)Å from the C(6)/N(1)/C(9)/C(8) plane.
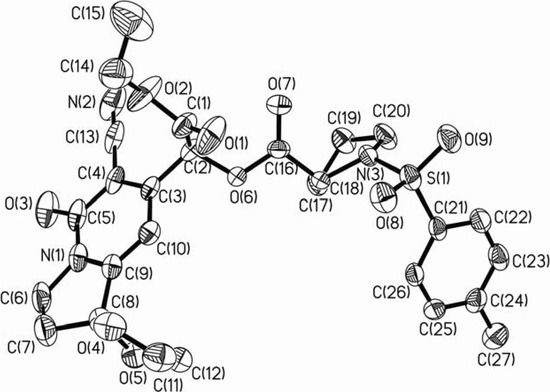
Figure 3.
ORTEP drawing and atom labelling scheme of compound 4a.
In order to explain the (2S, 17R)-configuration preference of the title compound crystallized from methanol, the total energies of compounds (2S, 17R)-4a and (2R, 17R)-4b were calculated using the HYPERCHEM program [7] at the semi-empirical PM3 computational level, where a root-mean-square gradient of the forces acting on each atom of 0.05 kcal/molÅ as the convergene criterion was employed. The final energies of 4a (-7332.73 a.u.) was slightly lower than that of 4b (-7327.60 a.u.). Thus, qualitatively, there is energy advantage for the indolizine analogue 4a. We conclude that there is a fast dynamic enol tautomerism equilibrium between compounds 4a and 4b (Figure 4). Upon changing the exterior condition such as heating, the equilibrium was destroyed and (2R, 17R)-4b was converted into the relatively stable (2S, 17R)-4a with lower energy.
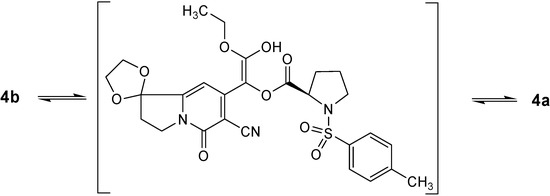
Figure 4.
Dynamic equilibrium of enol tautomerism between 4a and 4b
Conclusions
For the first time we have prepared optically active [N-tosyl-(R)-prolyloxy]-2(S)-[4-cyano-8,8-ethylenedioxy-5-oxo-5,6,7,8-tetrahydroindolizin-3-yl] acetate (4a) and characterized it by 2D-NMR spectroscopy and X-ray crystallography. Theoretical calculations have further confirmed the configurational analysis. From the point of view of the synthetic strategy and atomic economy, it is preferable if chiral centers are constructed as soon as possible in an asymmetric synthetic route. Use of optically active compound (2S, 17R)-4a as substrate instead of the usual mixture of diastereoisomers 4a and 4b, should help improve the stereoselectivity of the subsequent ethylation process. Further studies are under way to optimize the reaction conditions for this diastereoselective ethylation and to develop a more practical and scaled-up preparation of 20(S)-CPTs.
Experimental Section
General
Melting points were determined with a WRS-1B digital melting point apparatus and are uncorrected. 1H- and 13C-NMR spectra were recorded on a Bruker DMX (500 MHz) instrument in CDCl3 solutions using TMS as an internal standard. Chemical shifts are given in the δ-scale (ppm). Mass spectra (MS) was performed on a MAT95 instrument [electronic impact (EI) at 70 eV]. Optical rotation was recorded on a JASCO P-1020 polarimeter. Diastereoisomeric excess (d.e.) was detected on an XTerra® MS C18 column (2.1×150 mm, Ø5 μm) using a Waters 2695 separations module (eluent: CH3CN/H2O=55:45; λmax=225 nm).
Synthesis of ethyl [N-tosyl-(R)-prolyloxy]-2(S)-[4-cyano-8,8-ethylenedioxy-5-oxo-5,6,7,8-tetrahydro-indolizin-3-yl] acetate (4a)
The mixture of N-tosyl-(R)-proline (2.16 g, 8.04 mmol) and Na2CO3 (0.34 g, 3.25 mmol) in anhydrous DMF (20 mL) was stirred for 10 minutes under nitrogen at room temperature. The solution of 2-bromoindolizine analogue 3 (1.4 g, 3.66 mmol) dissolved in anhydrous DMF (10 mL) was added to the above reaction mixture and stirred for another 1.5 hours at 70 ˚C. The reaction mixture was cooled, diluted with water (100 mL) and extracted with ethyl acetate (4 × 50 mL). The combined organic phase was successively washed with 5% NaHCO3 solution (50 mL), water (50 mL), and brine (50 mL), then dried over MgSO4 and evaporated to dryness. The residue was crystallized from methanol (45 mL) to give 4a (1.46 g, 70%) with a melting point of 172.6-174.3 ˚C, [α]20D +140.62˚ (c 1, CHCl3), d.e.>99%. 1H- and 13C-NMR data see Table 1.
X-ray techniques
A block single crystal of the title compound 4a suitable for X-ray diffraction was grown by slow evaporation from a methanol solution. Crystal and experimental data are summarized in Table 2. The data were collected with a Bruker SMART CCD area-detector diffractometer equipped with a graphite-monochromatized Mo Kα radiation (λ = 0.71073Å) at 293(2) K. The crystal structure data have been deposited at the Cambridge Crystallographic Data Ceter [8]. Absorption corrections were made with semi-empirical from equivalents. The crystal structure was resolved by direct methods using SHELXS-97 [9a] and refined by full-matrix least-squares methods on F2 using SHELXL-97 [9b]. All non-H atoms were refined anisotropically.

Table 2.
Crystal and experimental data for compound 4a.
| Formula | C27H29N3O9S |
| Formula weight | 571.59 |
| Crystal system | Trigonal |
| Space group | P3(2) |
| Unit-cell dimensions (Å) | a = 10.303(2) |
| b = 10.303(2)22.564(6) | |
| c = 22.564(6) | |
| Unit-cell volume, V (Å3) | 2074.3(8) |
| Formula per unit cell, Z | 3 |
| Dcalcd (g/cm3) | 1.373 |
| Absorption coefficient, μ(mm-1) | 0.175 |
| F(000) | 900 |
| Crystal size (mm) | 0.20 × 0.10 × 0.08 |
| θ Range (°) | 2.28-26.00 |
| Index ranges | -12 ≤ h ≤ 12 |
| -12 ≤ k ≤ 10 | |
| -27 ≤ l ≤ 27 | |
| Max. and min. transmission | 0.9861 and 0.9658 |
| Independent reflection | 5345 (Rint = 0.0323) |
| Data/restraints/parameters | 5345 / 1 / 362 |
| Final R indices [I>2σ(I)] | R1 = 0.0679, wR2 = 0.1003 |
| R indices (all data) | R1 = 0.1067, wR2 = 0.1119 |
| Goodness-of-fit on F2 | 1.090 |
| Largest difference peak and hole (e/Å3) | 0.231 and -0.146 |
Acknowledgments
We are grateful to Shanghai Educational Development Foundation to sponsor this research project.
References and Notes
- Li, Q. Y.; Zu, Y. G.; Shi, R. Z.; Yao, L. P. Review camptothecin: current perspectives. Curr. Med. Chem. 2006, 13, 2021–2039. [Google Scholar] Du, W. Towards new anticancer drugs: a decade of advances in synthesis of camptothecins and related alkaloids. Tetrahedron 2003, 59, 8649–8687. [Google Scholar]
- Ejima, A.; Terasawa, H.; Sugimori, M.; Tagawa, H. Antitumour agents. Part 2. asymmetric synthesis of (S)-camptothecin. J. Chem. Soc. Perkin Trans. 1. 1990, 27–31. [Google Scholar] Ejima, A.; Terasawa, H.; Sugimori, M.; Tagawa, H. Asymmetric synthesis of (S)-camptothecin. Tetrahedron Lett. 1989, 30, 2639–2640. [Google Scholar]
- Jew, S. S.; Ok, K. D.; Kim, H. J.; Kim, M. G.; Kim, J. M.; Hah, J. M.; Cho, Y. S. Enantioselective synthesis of 20(S)-camptothecin using sharpless catalytic asymmetric dihydroxylation. Tetrahedron: Asymmetry 1995, 6, 1245–1248. [Google Scholar] [CrossRef]
- Imura, A.; Itoh, M.; Miyadera, A. Enantioselective synthesis of 20(S)-camptothecin using an enzyme-catalyzed resolution. Tetrahedron: Asymmetry 1998, 9, 2285–2291. [Google Scholar] Miyadera, A.; Imura, A. Optical resolution of indolizines with bacillus species. JP 04200393, 1990. [Google Scholar] Terasawa, H.; Sugimori, M.; Tagawa, H.; Ejima, A. Antitumor agents. III. A novel procedure for inversion of the configuration of a tertiary alcohol related to camptothecin. Chem. Pharm. Bull. 1989, 37, 3382–3385. [Google Scholar]
- Chen, F. E.; Kuang, Y. Y. Preparation of 2',3'-dihydro-5,5,7'-trimethyl-5'-oxo-spiro[1,3-dioxane-2,1'(5'H)-indolizine]-6'-carbonitrile. CN 1651434, 2005. [Google Scholar] Chen, F. E.; Zhang, L. P.; Kuang, Y. Y. Synthesis of ethyl 2-ethyl-2-[6-cyano-1,1-(ethylenedioxy)-5-oxo-1,2,3,5-tetrahydro-indolizine] acetate. CN 1858051, 2006. [Google Scholar] Huo, M.; Kuang, Y. Y.; Chen, F. E. Practical route to a β-ketophosphonate, a key intermediate for the total synthesis of 20(S)-CPT and related analogues. Org. Prep. Proc. Int. 2004, 36, 331–335. [Google Scholar] Kuang, Y. Y.; Huo, M.; Chen, F. E. 3-Bromomethyl-3-ethyl-3,4,6,7,8,8a-hexahydro-1H-pyrrolo[2,1-c][1,4]-oxazine-1,4-dione. Acta Cryst. 2004, C60, o505–506. [Google Scholar] Kuang, Y. Y.; Ji, L.; Chen, F. E. A convenient and efficient asymmetric synthesis of (S)-α-arylthiomethyl-α-hydroxybutyric acid esters. Org. Prep. Proc. Int. 2005, 37, 119–123. [Google Scholar]
- Shanghai No. 5 and No. 12 Pharmaceutical Plant, Shanghai Institute of Pharmaceutical Industrial Research and Shanghai Institute of Materia Medica. Total synthesis of dl-camptothecin. Chin. Sci. Bull. 1977, 6, 625–634. [Google Scholar] Wani, M. C.; Ronman, P. E.; Lindley, J. T.; Wall, M. E. Plant antitumor agents. 18. Synthesis and biological activity of camptothecin analogues. J. Med. Chem. 1980, 23, 554–560. [Google Scholar]
- Hypercube. HYPERCHEM, release 5.0; Hypercube Inc.: Gainesville, FL, USA, 1998. [Google Scholar]
- CCDC 648359 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).
- Sheldrick, G. M. SHELXS97, Programs for the solution of crystal structure; Univ. of Göttingen: Göttingen, Germany, 1997. [Google Scholar] Sheldrick, G. M. SHELXL97, Programs for the refinement of crystal structure; Univ. of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sample Availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.