Introduction
Daidzein (7,4-dihydroxyisoflavone) is one of the major isoflavones found in a wide variety of plant-derived foods, especially in soybeans and soy-based foods. Belonging to the isoflavone subclass of flavonoids, daidzein has a flavone nucleus composed of two benzene rings linked through a heterocyclic pyrone ring [
1]. Because of the structural similarity to natural estrogens (
Figure 1), daidzein can bind estrogen receptors (ERs) and induce ER-dependent DNA binding and activation of estrogen responsive promoters in many cell types [
2]. There are two types of estrogen receptor (ERα and ERβ), both of which are expressed in the brain, and daidzein exhibits greater affinity towards ERβ than to ERα [
3]. Although a variety of epidemiological,
in vivo and
in vitro experiments have linked soy-derived isoflavones with positive Central Nervous System effects [
4,
5,
6,
7], few
in vivo studies have assessed whether chronic administration of daidzein produces anti-apoptosis effects, and the mechanism of its neuroprotective effects is not fully understood.
Figure 1.
The structures of 17β-estradiol and daidzein.
Figure 1.
The structures of 17β-estradiol and daidzein.
Neurodegeneration is a complex process involved in the decline of various physiological functions, which may be caused by cell death. During neurodegeneration the rate of cell death is elevated in postmitotic cells. Especially in the neurodegeneration of the nervous system, many of the dying neurons exhibit signs of apoptosis which might occur as a result of oxidative stress [
8,
9]. Two major regulatory pathways have been described in apoptosis: one is the intrinsic, in which mitochondria play a central role; and the other is the extrinsic, in which cell plasma membrane receptors act as the starting point of the apoptotic process. In both the intrinsic and extrinsic pathways, activation of caspases plays an indispensable role [
10].
The bcl-2 family proteins play an important role in regulating the apoptotic signaling pathway involving mitochondria [
11]. This family of proteins can be separated into two functionally distinct groups: anti- and pro-apoptotic proteins [
12]. Bcl-2 is a 25 kDa anti-apoptotic protein [
13,
14], decreasing its expression with aging [
15]. However, bax is a pro-apoptotic member of bcl-2 family, which is 21 kDa and promotes cell death by forming a heterodimer with bcl-2 and blocking its anti-apoptotic actions [
16,
17,
18]. Caspase-3, is a pivotal enzyme in all the apoptotic pathways. It is generally synthesized in cells as an inactive zymogene (32 kDa) and requires proteolytic activation. After the activation, it is cleaved at the carboxyl sides of two specific aspartic acid residues to form two mature subunits, p18 (18 kDa) and p12 (12 kDa)[
19]. Caspase-3 activation is essential for certain processes associated with the dismantling of the cell and the formation of apoptotic bodies [
20].
The increasing evidence of neuromodulatory role of phytoestrogens on the nervous system, in particular, raises the possibility that phytoestogen might alter risk for neurodegeneration through its effect on apoptosis. To contribute to the knowledge of neuroprotective effect of daidzein on the apoptosis pathway, we examined the effect of daidzein on the expression of bcl-2 mRNA, bax mRNA and capase-3 in hippocampus and cortex regions of the brain.
Results and Discussion
Daidzein, a naturally occurring plant chemical with estrogen-like structure and action, is currently receiving considerable attention due to its favourable effects in the therapy of some cancers, osteoporosis, chronic renal disease, atherosclerosis and coronary heart disease. Nowadays, soya phytoestrogens have also been shown to act like estrogen in the brain, which has direct and indirect receptor-mediated genomic effects that could affect cognition, cell survival, growth and neuroplasticity.
D-Galactose (D-gal) has been used as an animal aging model for brain aging or in anti-aging pharmacology research. Behavior tests showed that that D-galactose can induce behavioural impairment in C57 mice [
12]. Although the exact mechanism underlying D-galactose-induced rodent aging has not been defined, existing data indicate that the oxidative stress induced by increased malondialdehyde and reduced glutathione peroxides and superoxide dismutase in the brain might be the main reason [
21]. However sustained perturbation induced by oxidative stress may result in apoptosis [
22]. Our results also indicated that D-gal could induce apoptosis in the mouse brain.
Neuronal apoptosis, with the involvement of caspases, can occur via the intrinsic pathway, which is controlled by bcl-2 family proteins [
23]. Bcl-2 may function as a counteracting force to reduce damage by reducing lipid peroxidation triggered by cytotoxic stimuli such as oxidative stress or by maintaining glutathione levels. Bcl-2 was also found to prevent the release of mitochondrial apoptotic factors including cytochrome c [
24], which combines with apoptosis protease-activating factor (Apaf-1) in the presence of adenosine triphosphate (ATP) to activate caspase-9 that, in turn, activates effector caspases such as caspase-3 [
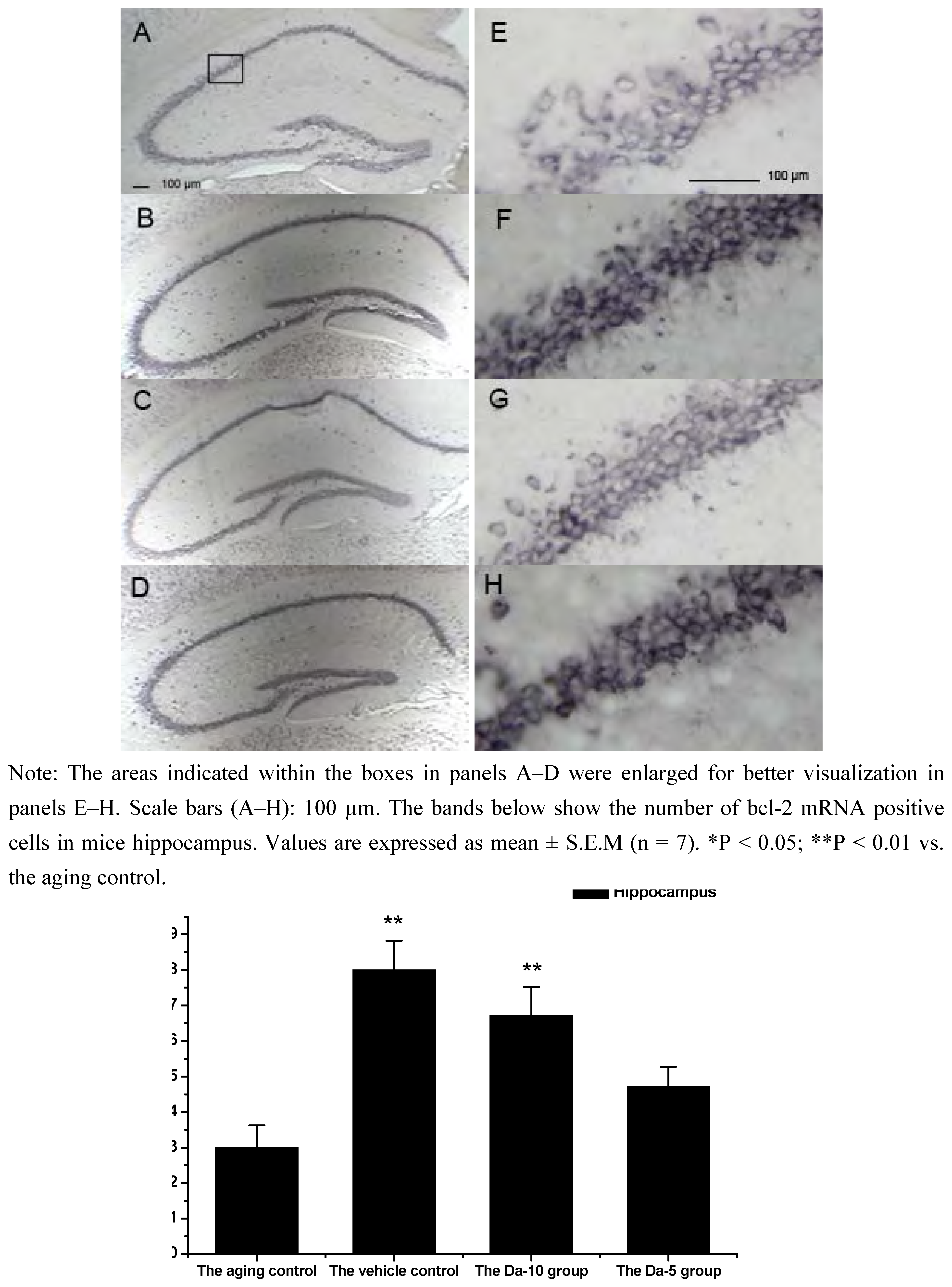
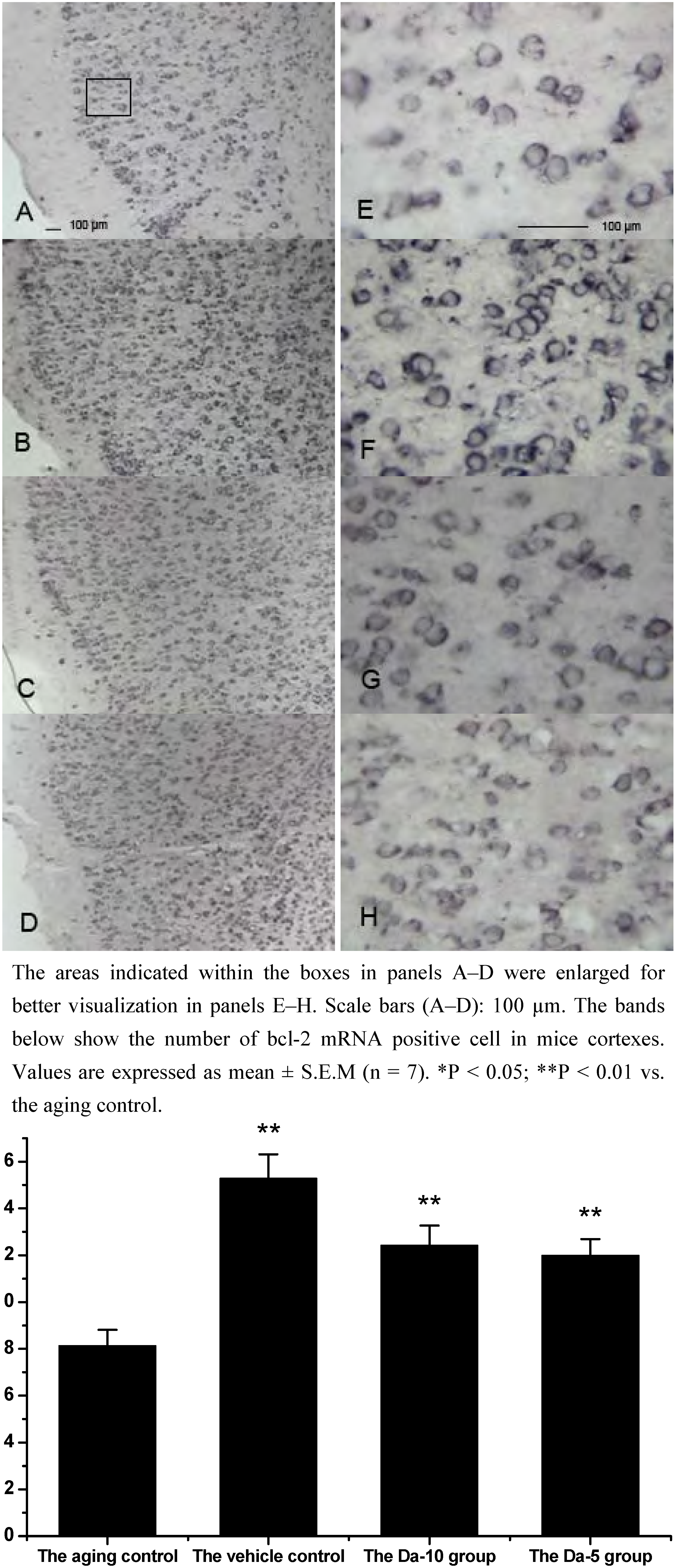
25]. In our experiment, Bcl-2 mRNA was found in all the regions analyzed in hippocampus (CA1, CA2, CA3, and dentate gyrus) and cortex. In both regions, daidzein could help increase the transcriptions of bcl-2 in D-gal treated mice brain. Briefly, in the hippocampus, a higher dose of daidzein (Da-10 group) showed a clear increase of bcl-2 mRNA positive cells (P<0.01) compared with the aged control; while at a lower dose of daidzein (Da-5 group), little bcl-2 mRNA expression change was noted (P>0.05) in the hippocampus. And in the cortex, the bcl-2 mRNA positive cell numbers in Da-10 group (P<0.01) and in Da-5 group (P<0.01) still tended to be significantly higher than that in the aged control (see
Figure 2 and
Figure 3).
Figure 2.
Effects of daidzein on the expression levels of bcl-2 mRNA assessed by non-radioactive in situ hybridization in D-gal-treated mice hippocampus. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Figure 2.
Effects of daidzein on the expression levels of bcl-2 mRNA assessed by non-radioactive in situ hybridization in D-gal-treated mice hippocampus. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Figure 3.
Effects of daidzein on the expression levels of bcl-2 mRNA assessed by non-radioactive in situ hybridization in D-gal-treated mice cortexes. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Figure 3.
Effects of daidzein on the expression levels of bcl-2 mRNA assessed by non-radioactive in situ hybridization in D-gal-treated mice cortexes. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
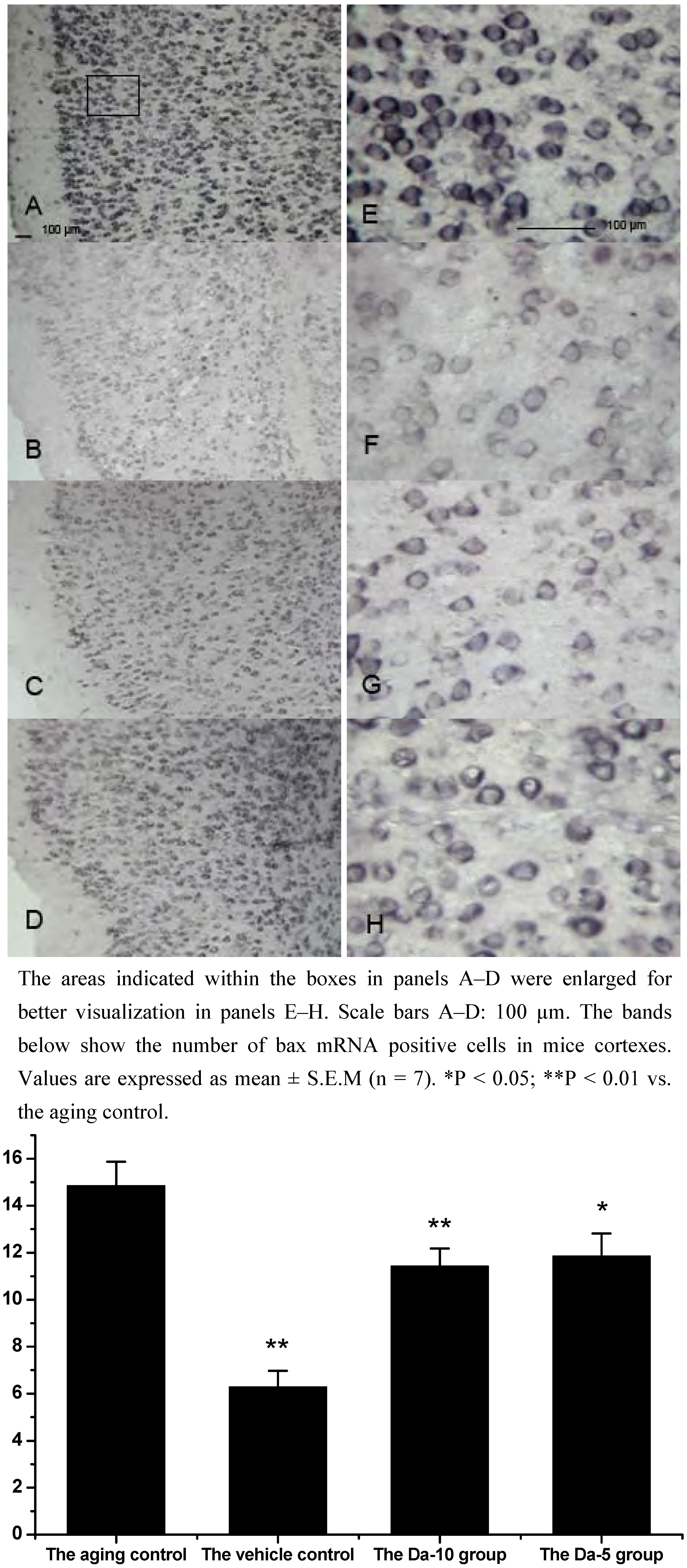
As a an pro-apoptotic member of bcl family, bax regulates apoptosis, not only by dimerizing with antiapoptotic Bcl-2 proteins, but also by regulating cytochrome c release and subsequent caspase activation [
26]. Since bcl-2 and bax could interact and regulate the activation of caspases, which could lead to apoptosis, the ratio of bax to bcl-2 was regarded as the cells to apoptosis index [
27,
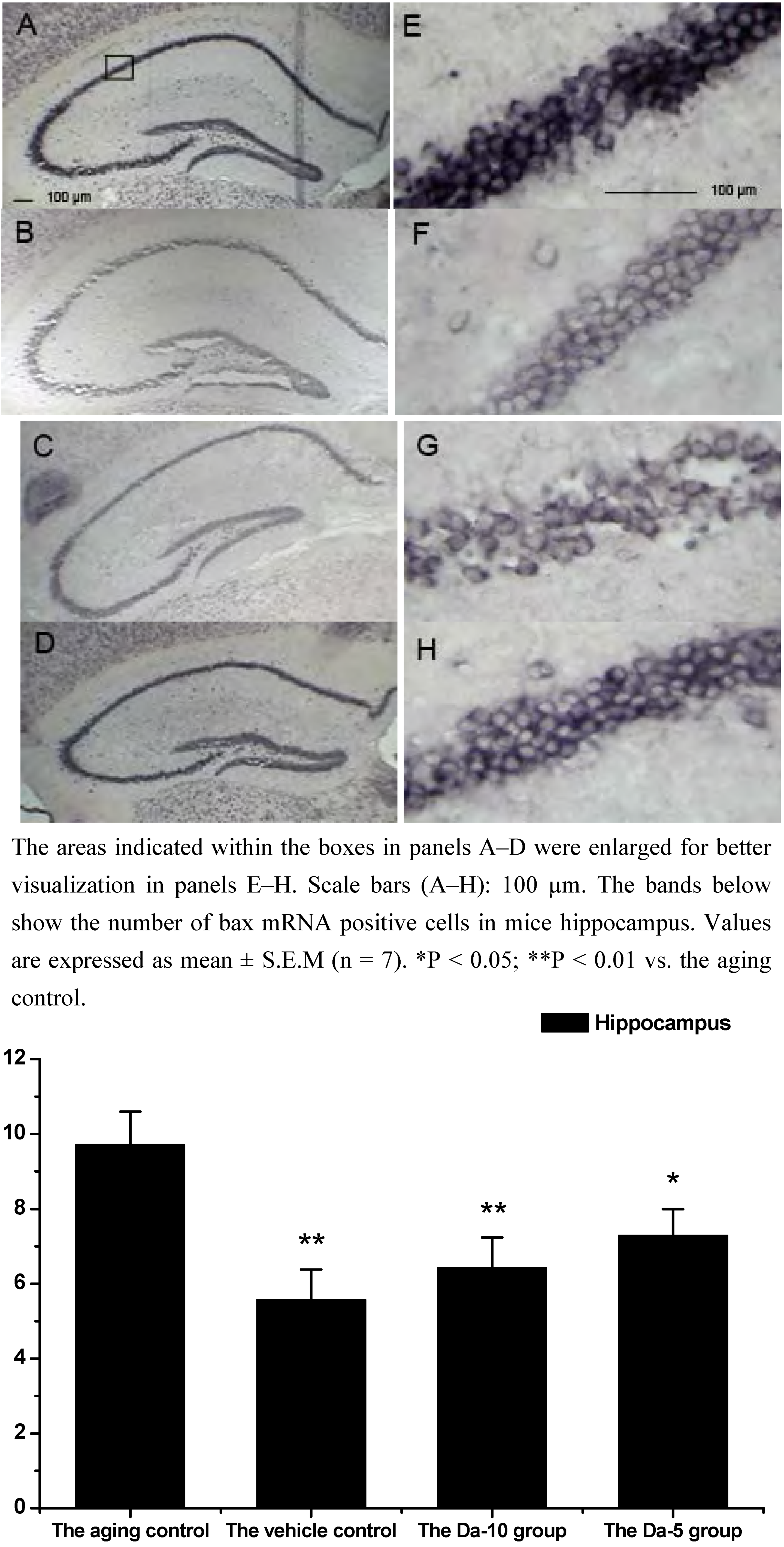
28]. Our results indicated that daidzein could affect the D-gal induced bax mRNA increase in both the hippocampus and cortex. Concretely, with treatment of daidzein, the positive cell numbers of bax mRNA in the Da-10 group (P<0.01) and Da-5 group (P<0.05) decreased significantly, compared with the aged control in hippocampus. In cortexes the positive cell numbers of bax mRNA in Da-10 group (P<0.01) and Da-5 group (P<0.05) were also decreased, compared with the aged control (see
Figure 4 and
Figure 5).
Figure 4.
Effects of daidzein on the expression levels of bax mRNA assessed by non-radioactive in situ hybridization in D-gal-treated mice hippocampus. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Figure 4.
Effects of daidzein on the expression levels of bax mRNA assessed by non-radioactive in situ hybridization in D-gal-treated mice hippocampus. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Figure 5.
Effects of daidzein on the expression levels of bax mRNA assessed by non-radioactive in situ hybridization in D-gal-treated mice cortexes. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Figure 5.
Effects of daidzein on the expression levels of bax mRNA assessed by non-radioactive in situ hybridization in D-gal-treated mice cortexes. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Results of the bcl-2 and bax analyses in our experiments indicate that daidzein could prevent neurons from triggering apoptosis via enhancement of the expression of bcl-2 mRNA and supression of bax mRNA expression in the hippocampus and cortex.
Caspases are a family of proteases with the characteristic that they cleave at aspartate residues in their substrates [
10]. Among them, caspase-3 acts as a key executor protease in apoptosis. Acculmulating evidence indicates that the expression of caspase-3 is increased in neurodegeneration brain [
29]. Pratima Sur found estrogen pretreatment decreased levels of caspase-3 in H
2O
2 treated C6 (rat glioma) cells [
30]. It was also demonstrated that genistein, an analog of daidzein, could suppress the activation of caspase-3 induced by Aβ25-35 via the ER-associated mechanism at a low concentration [
7].
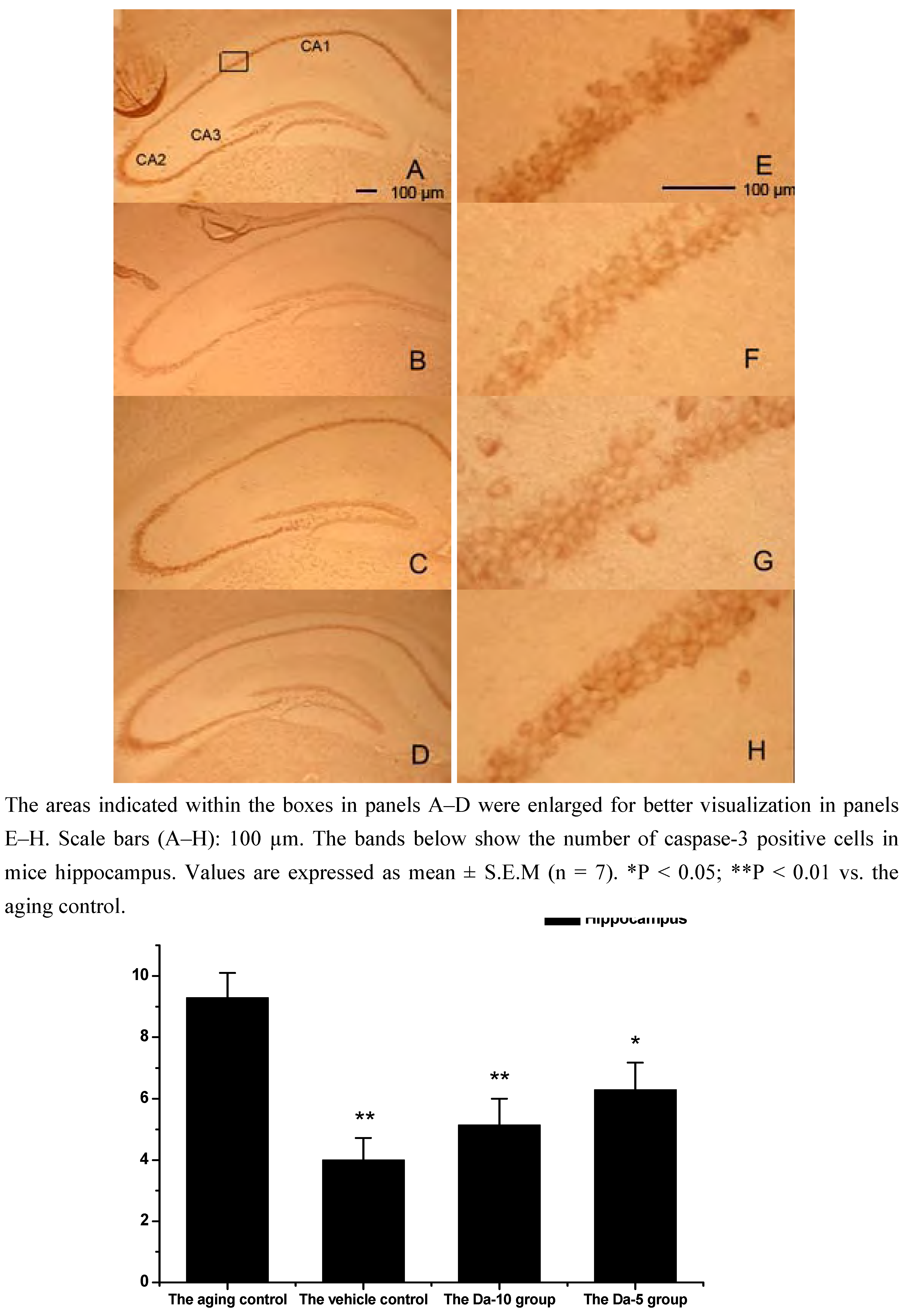
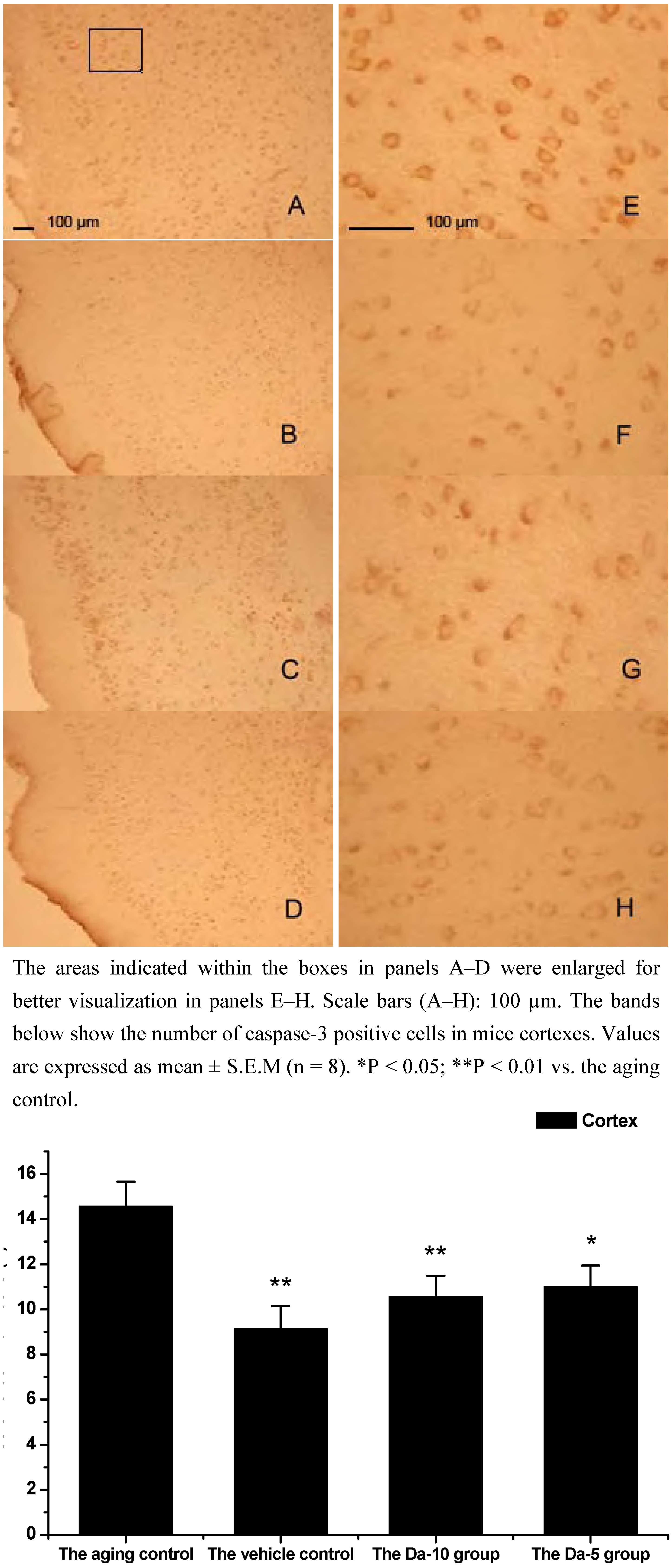
This is consistent with our results showing that daidzein could decrease the expression of caspase-3 in hippocampus and cortexes of the D-gal treated mice. Concretely, compared with the aged control, daidzein could decrease the caspase-3 expression in the cortex (P<0.05) and hippocampus (P<0.05) in the Da-5 group. In the Da-10 group this inhibition of daidzein in caspase-3 expression was more intensively in the cortex (P<0.01) or in the hippocampus (P<0.01) (See
Figure 6 and
Figure 7).
Figure 6.
Effects of daidzein on the expression levels of caspase-3 in D-gal-treated mice hippocampus. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Figure 6.
Effects of daidzein on the expression levels of caspase-3 in D-gal-treated mice hippocampus. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Figure 7.
Effects of daidzein on the expression levels of caspase-3 in D-gal-treated mice cortexes. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Figure 7.
Effects of daidzein on the expression levels of caspase-3 in D-gal-treated mice cortexes. (A) Aging control. (B) Vehicle control. (C) Da-10 group. (D) Da-5 group.
Several studies have shown age-related changes in the levels of proteins and factors that regulate apoptosis [
31]. Estrogen therapy protects against age-related cell death by influencing the expression of genes that suppress apoptotic cell death pathways [
32]. Daidzein can act as a partial estrogen agonist, it can bind to ERs and interact with estrogen response elements (EREs) located within the regulatory region of target genes, and then lead to either activation or suppression of transcription of a target gene [
2], such as bcl-2 [
33]. Regulations of bcl-2 family protein also affect the activation of caspase-3 [
34]. From our observations, a decrease in caspase-3 expression correlates well with a decrease in bax mRNA and an increase in bcl-2 mRNA expression, as the bcl-2 family protein is thought to be upstream of caspases in the mitochondrial-mediated apoptotic pathway.
Experimental
Animals
Eight-week-old female Kunming strain mice (28.76±3.32 g, obtained from the Branch of National Breeder Center of Rodents, Shanghai, P.R. China) were kept under standard laboratory conditions with food and water ad libitum, and maintained on a 12 h light/dark cycle. After a one week adaptation period, the mice were randomly divided into four groups: the vehicle control, the D-gal model, Da-5 group and Da-10 group. For the first eight weeks, the vehicle control group received only vehicle (0.9 % saline), and the other three groups received 50 mg D-gal (Sigma-Aldrich, MO, USA) per kilogram body weight, respectively, by daily subcutaneous injection. Then for the next six weeks, daidzein (99%, provided by Professor Chen Jie, Nanjing Agricultural University, Nanjing, P.R. China) was dissolved in distilled water containing 0.1% Tween-80 and administered orally to two groups of D-gal induced aging mice at 5 and 10 mg/kg day (Da-5 group and Da-10 group, respectively). Meanwhile, another group of D-gal-induced aging mice (the D-gal model) and the vehicle control were given distilled water (dd H2O/0.1% Tween-80) without daidzein. All experiments were carried out in accordance with the Chinese legislation on the use and care of laboratory animals and were approved by the respective university committees for animal experiments. Efforts were made to minimize animal suffering and to reduce the number of animals used. Mice were sacrificed 24 h after the last treatment, and brain tissues were immediately collected for experiments or stored at -70°C for later use.
Preparation of tissue samples
The mice were perfused transcardially with normal saline (0.9 %, 25 mL) and brain tissues were fixed in a fresh solution of 4% paraformaldehyde (pH 7.4, Sigma) for 2 hours, incubated overnight at 4 °C in 30% sucrose contained 100 mM sodium phosphate buffer (pH 7.4), then embedded in Optimal Cutting Temperature Cryoembedding Matrix (OCT, Leica Microsystems, Bensheim, Germany). Cryosections (12 μm) were collected on 3-aminopropyltrimethoxysilane-coated slides (Sigma) and stored at -70 °C.
In situ hybridization
Probes were generated from mouse brain cDNA using specific PCR primers. AMV reverse transcriptase (Promega), and oligo-(dT) 15 primers (Promega) were used for generating total cDNA. PCR cloning of Bcl-2 and Bax was performed with primers containing EcoRI (New England Biolabs) and Hind III (New England Biolabs) restriction enzyme cut site. The Bcl-2 and Bax sequences of PCR primers were as follows:
Bcl-2: 5’-CCCCTGGCATCTTCTCCTTCC-3’, 5’-GGGTGACATCTCCCTGTGACG-3’.
Bax: 5’-GGATGCGTCCACCAAGAA-3’, 5’-ACGGAGGAAGTCCAGTGT-3’.
PCR was performed on a Hybaid Omnigene thermal cycler using the following conditions: [94°C (4 min) 1 cycle]; [94°C (1 rain), 56°C (1 min), 72°C (1 min) - 35 cycles]; [72°C (10 min) - 1 cycle]. The PCR reaction contained 2.5% of the cDNA product, 10 mM Tris-HCI (pH 9), 50 mM KC1, 0.01% Triton X-100 (Promega), 5 µM of each primer, 0.1 mM dNTPs, 1.5 mM MgCl2 and 1.5 U Taq polymerase (Promega) in a final volume of 25 µL. A single PCR product was observed in each case by gel electrophoresis, and was purified using PCR Clean-Up System kit (Promega) and then cloned directly into pGEM3Z (Promega). The sequence and orientation of the inserts were confirmed by automated sequencing.
Plasmids were linearized with BamHI or EcoRI for transcription with T7 or SP6 RNA polymerase to generate sense and antisense riboprobes. Antisense and sense (control) riboprobes were labeled by in vitro transcription in a reaction mixture containing DIG RNA Labeling mix (Roche), 1 µg of linearized plasmids and 20 units of SP6 or T7 RNA polymerase (Promega) according to manufacturer’s protocol. After transcription, the template DNA was digested with 40 units DNase I for 15 min at 37 °C. Unincorporated labeling molecules were removed by centrifugation.
The in situ hybridization was carried out as described (Roche Diagnostics Corporation, Indianapolis, IN, USA), with all steps being performed under RNAse-free conditions prior to hybridization. The number of positive cells in 0.01 mm2 was estimated by blind manual counting of seven regions located at a consistent position per section. Cell densities were calculated by dividing the cell numbers by the measured counting area (cells/mm2). Seven regions were estimated at a consistent position per section by blind manual counting.
Immunohistochemistry
For immunohistochemistry, endogenous peroxide activity in the sectioned tissues was blocked with 3% H2O2, and non-specific binding sites were blocked with 3% normal goat serum (Chemicon International Inc., Temecula, CA, USA). The sections were incubated overnight with primary antibody for cleaved caspase-3 (Cell Signaling Technology Inc., Beverly, MA, USA) at the concentration of 1:100. Anti-Rabbit HRP EnvisionTM System, (Dako, Glostrup, Denmark) were used as secondary antibody. The sections were incubated with DAB solution (Dako), then washed thoroughly in tap water for the observation by the microscope (Leica Microscopy Systems, Heidelberg, Germany). The number of caspase-3 positive cells in 0.01 mm2 was estimated by blind manual counting of seven regions located at a consistent position per section.
Statistical analysis
Results were shown on the mean ± S.E. Statistical significance between groups was analyzed by one-way ANOVA followed by the Student–Newman–Keuls Multiple Comparisons tests. P-values less than 0.05 were considered significant.