Two Step Synthesis of a Non-symmetric Acetylcholinesterase Reactivator
Abstract
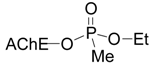
:Introduction
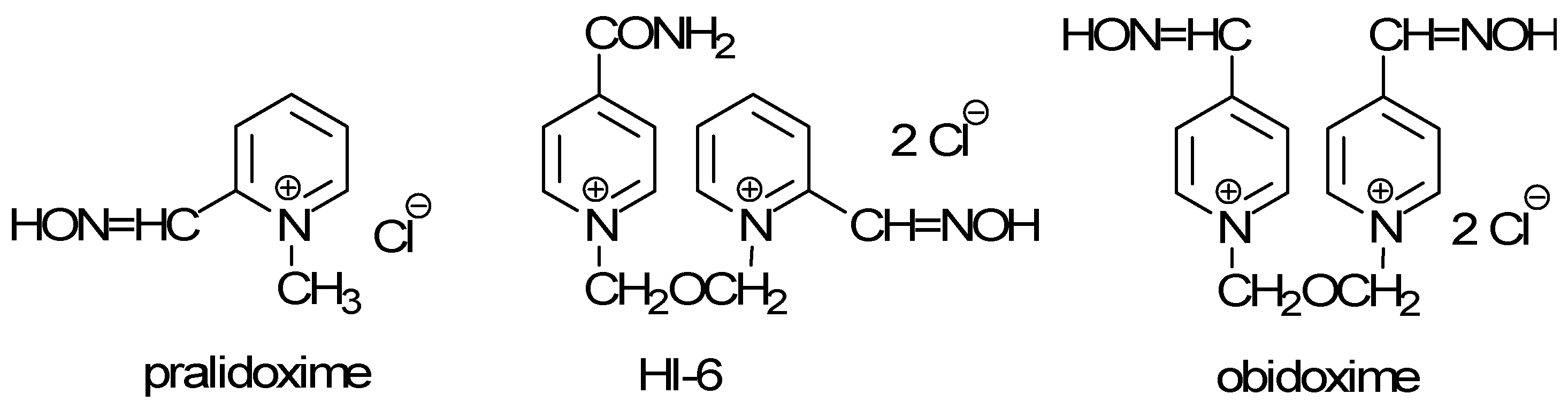
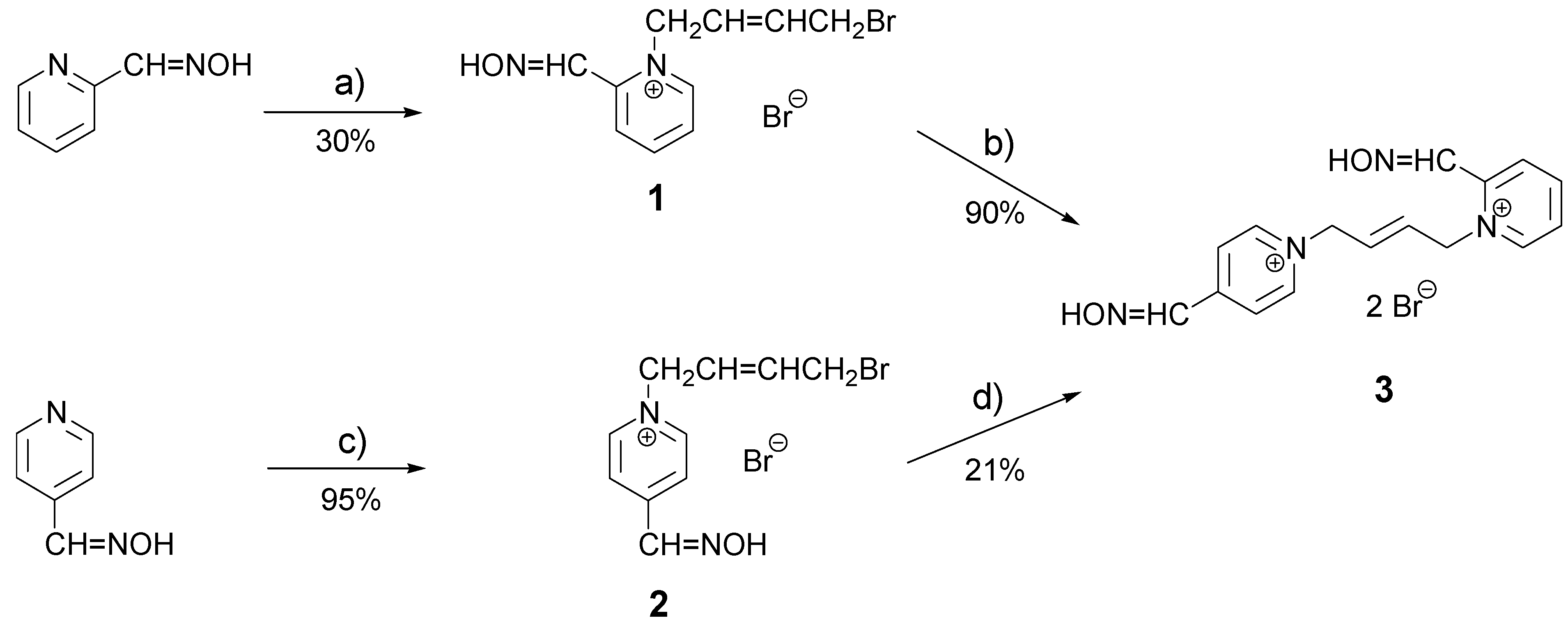
Results and Discussion
Biochemistry
| Chlorpyrifos |  | 63 | 48 |
| Tabun | 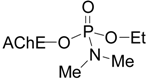 | 9 | 0 |
| Cyclosarin | 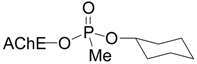 | 31 | 40 |
Conclusions
Experimental
General
Synthesis
Product characterization data
Acknowledgements
References
- Marrs, T.C. Organophosphate poisoning. Pharmacol. Therap. 1993, 58, 51–66. [Google Scholar] [CrossRef]
- Bajgar, J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004, 38, 151–216. [Google Scholar] [PubMed]
- Kuca, K.; Jun, D.; Musilek, K. Structural requirements of acetylcholinesterase reactivators. Mini-Rev. Med. Chem. 2006, 6, 269–277. [Google Scholar]
- Pang, YP.; Kollmeyer, TM.; Hong, F.; Lee, JC.; Hammond, PI.; Haugabouk, SP.; Brimijoin, S. Rational design of alkylene-linked bis-pyridiniumaldoximes as improved acetylcholinesterase reactivators. Chem. Biol. 2003, 10, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Musilek, K.; Kuca, K.; Jun, D.; Dohnal, V.; Dolezal, M. Synthesis of the novel series of bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. Biorg. Med. Chem. Lett. 2006, 16, 622–627. [Google Scholar]
- Kim, T.H.; Kuca, K.; Jun, D.; Jung, Y.S. Design and synthesis of new bis-pyridinium oximes as cyclosarin-inhibited acetylcholinesterase reactivators. Bioorg. Med. Chem. Lett. 2005, 15, 2914–2917. [Google Scholar] [CrossRef] [PubMed]
- Musilek, K.; Holas, O.; Hambalek, J.; Kuca, K.; Jun, D.; Dohnal, V.; Dolezal, M. Synthesis of Bispyridinium Compounds bearing Propane Linker and Evaluation of their Reactivation Activity against Tabun- and Paraoxon-Inhibited Acetylcholinesterase. Lett. Org. Chem. 2006, 3, 831–835. [Google Scholar] [CrossRef]
- Musilek, K.; Kuca, K; Jun, D.; Dolezal, M. Progress in synthesis of new acetylcholinesterase reactivators during the period 1990-2004. Curr. Org. Chem. 2007, 11, 229–238. [Google Scholar]
- Kuca, K.; Bielavsky, J.; Cabal, J.; Bielavska, M. Synthesis of a potential reactivator of acetylcholinesterase 1-(4-hydroxyiminomethylpyridinium)-3-(carbamoylpyridinium)-propane dibromide. Tetrahedron Lett. 2003, 44, 3123–3125. [Google Scholar]
- Kuca, K.; Kassa, J. In vitro reactivation of acetylcholinesterase using of the oxime K027. Vet. Hum. Toxicol. 2004, 46, 15–18. [Google Scholar] [PubMed]
- Kassa, J.; Kuca, K.; Cabal, J.; Paar, M. A comparison of the efficacy of new asymmetric bispyridinium oximes (K027, K048) with currently available oximes against tabun by in vitro and in vivo methods. J. Toxicol. Env. Health 2006, 69, 1875–1882. [Google Scholar]
- Calic, M.; Lucic-Vrdoljak, A.; Radic, B.; Jelic, D.; Jun, D.; Kuca, K.; Kovarik, Z. In vitro and in vivo evaluation of pyridinium oximes: mode of interaction with acetylcholinesterase, effect on tabun- and soman-poisoned mice and their cytotoxicity. Toxicology 2006, 219, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Petroianu, GA.; Arafat, K.; Kuca, K.; Kassa, J. Five oximes (K-27, K-33, K-48, BI-6 and methoxime) in comparison with pralidoxime: in vitro reactivation of red blood cell acetylcholinesterase inhibitied by paraoxon. J. Appl. Toxicol. 2006, 26, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Petroianu, GA.; Nurulain, SM.; Nagelkerke, N.; Al-Sultan, MAH.; Kuca, K.; Kassa, J. Five oximes (K-27, K-33, K-48, BI-6 and methoxime) in comparison with pralidoxime: survival in rats exposed to the organophosphate paraoxon. J. Appl. Toxicol. 2006, 26, 262–268. [Google Scholar]
- Tekes, K.; Hasan, MY.; Sheen, R.; Kuca, K.; Petroianu, G. HPLC determination of the serum concentration of K-27, a novel oxime-type cholinesterase reactivator. J. Chromatogr. A. 2006, 1122, 84–87. [Google Scholar] [CrossRef]
- Lucic-Vrdoljak, A.; Calic, M.; Radic, B.; Berend, S.; Kuca, K.; Kovarik, Z. Pre-treatment with pyridinium oximes improves antidotal therapy against tabun poisoning. Toxicology 2006, 228, 41–50. [Google Scholar]
- Gupta, R. Toxicology of organophosphate & carbamate compounds. In Cl Cholinesterase inhibitors as chemical warfare agents: Community preparedness guidelines; Elsevier Academic Press: London, 2006; pp. 47–68. [Google Scholar]
- Kuca, K.; Jun, D.; Musilek, K.; Bajgar, J. Structure-activity relationship for the reactivators of acetylcholinesterase inhibited by nerve agent VX. Toxicol. Lett. 2006, 164, S51–S52. [Google Scholar] [CrossRef]
- Kuca, K.; Cabal, J. Evaluation of newly synthesized reactivators of the brain cholinesterase inhibited by sarin-nerve agent. Toxicol. Mech. Meth. 2005, 15, 247–252. [Google Scholar]
- Sample Availability: Samples of the prepared compounds are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Musilek, K.; Kuca, K.; Dohnal, V.; Jun, D.; Marek, J.; Koleckar, V. Two Step Synthesis of a Non-symmetric Acetylcholinesterase Reactivator. Molecules 2007, 12, 1755-1761. https://doi.org/10.3390/12081755
Musilek K, Kuca K, Dohnal V, Jun D, Marek J, Koleckar V. Two Step Synthesis of a Non-symmetric Acetylcholinesterase Reactivator. Molecules. 2007; 12(8):1755-1761. https://doi.org/10.3390/12081755
Chicago/Turabian StyleMusilek, Kamil, Kamil Kuca, Vlastimil Dohnal, Daniel Jun, Jan Marek, and Vit Koleckar. 2007. "Two Step Synthesis of a Non-symmetric Acetylcholinesterase Reactivator" Molecules 12, no. 8: 1755-1761. https://doi.org/10.3390/12081755