Palladate Salts from Chiral Pyridinium Ionic Liquids: Synthesis and Crystal Structures
Abstract
:Introduction
Results and Discussion
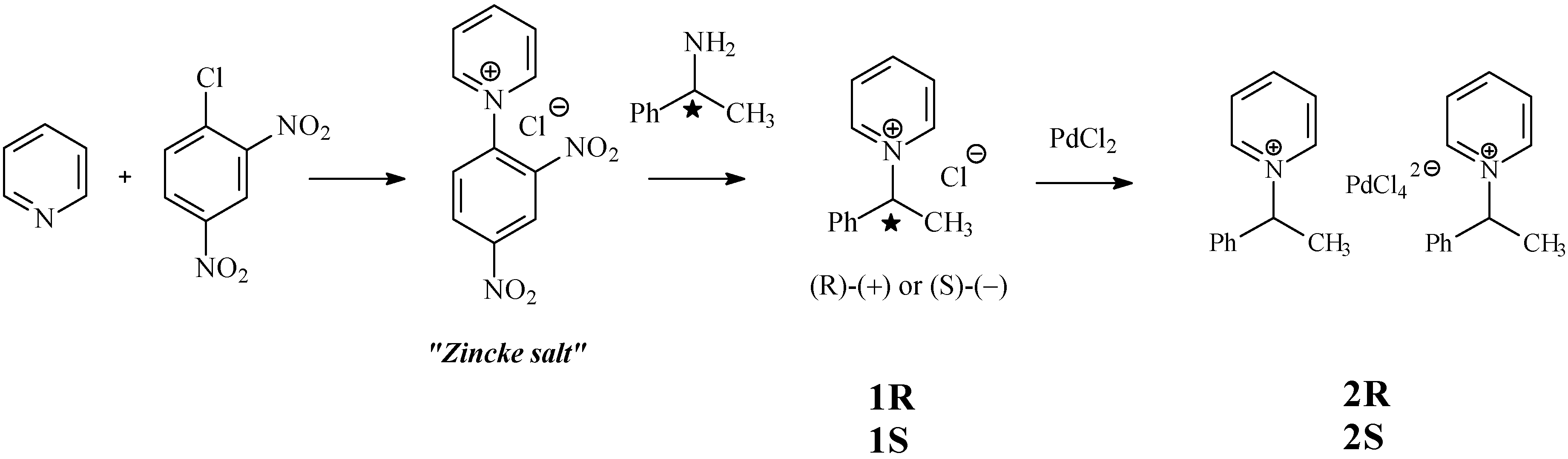

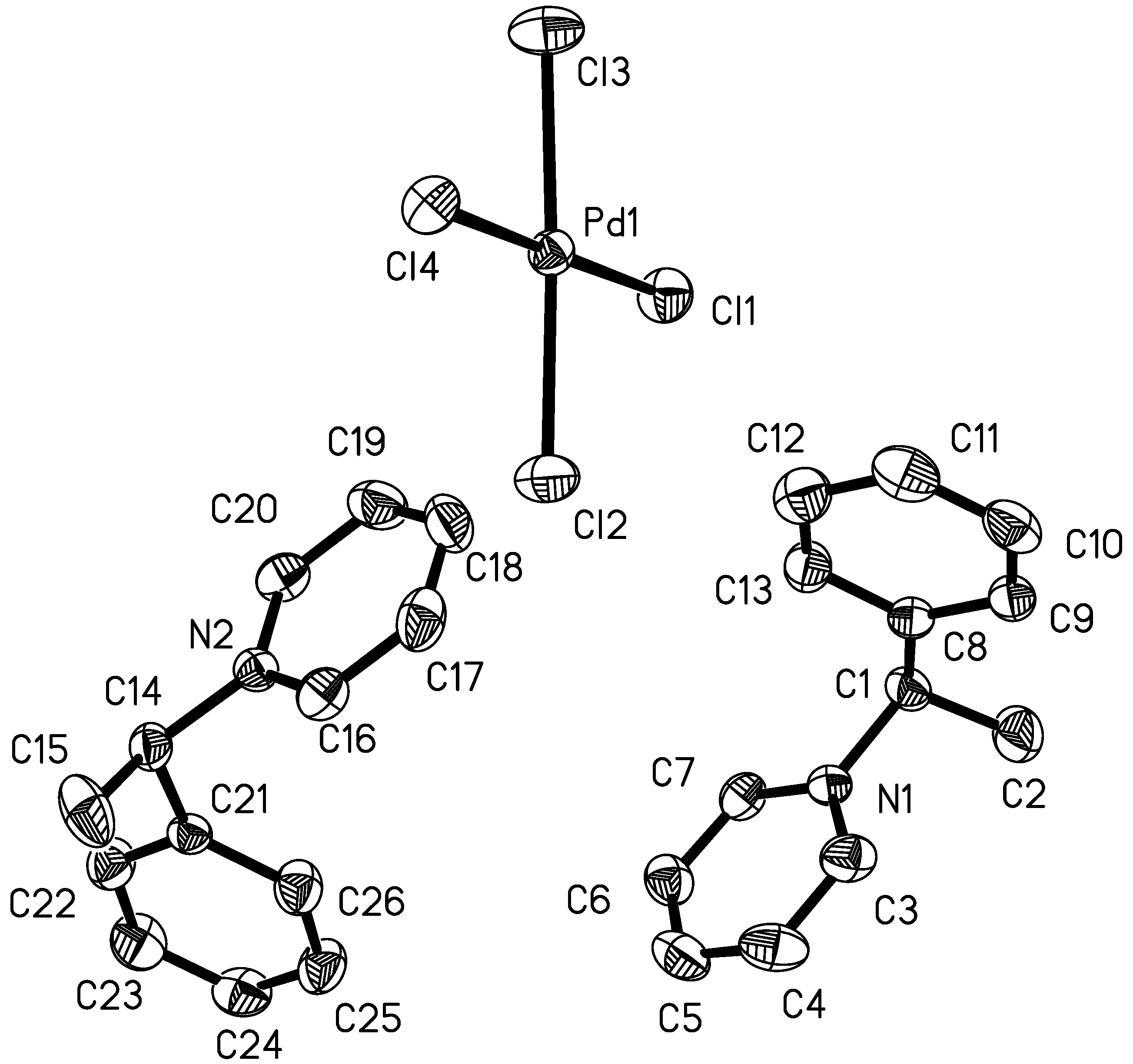
Conclusions
Experimental
General
Synthesis of (R)– or (S)–1-phenylethylpyridinium chloride (1R and 1S)
Synthesis of (R)– or (S)–bis(1-phenylethylpyridinium) tetrachloropalladate (2R and 2S)
Crystal data for 1R and 2R
| Identification code | [py*][Cl] (1R) | [py*]2[PdCl4] (2R) |
| Empirical formula | C13H14.50ClNO0.25 | C26H28Cl4N2Pd |
| Formula weight | 224.21 | 616.70 |
| Temperature | 193(2) K | 173(2) K |
| Wavelength | 0.71073 Å | 0.71073 Å |
| Crystal system | Tetragonal | Orthorhombic |
| Space group | P41 | P212121 |
| Unit cell dimensions | a = 22.369(1) Å | a = 8.059(1) Å |
| b = 22.369(1) Å | b = 12.211(1) Å | |
| c = 9.570(1) Å | c = 27.872(2) Å | |
| Volume | 4788.9(4) Å3 | 2742.7(3) Å3 |
| Z | 16 | 4 |
| Density (calculated) | 1.244 Mg/m3 | 1.494 Mg/m3 |
| Absorption coefficient | 0.29 mm-1 | 1.08 mm-1 |
| F(000) | 1896 | 1248 |
| Crystal size | 0.5 x 0.6 x 0.6 mm3 | 0.1 x 0.1 x 0.5 mm3 |
| Theta range for data collection | 5.1 to 23.3°. | 1.5 to 26.4°. |
| Index ranges | -24<=h<=24
-23<=k<=24 -10<=l<=8 | -10<=h<=9 -12<=k<=15 -30<=l<=34 |
| Reflections collected | 21441 | 16203 |
| Independent reflections | 6428 [R(int) = 0.0184] | 5600 [R(int) = 0.0225] |
| Completeness to theta | (=23.3°) 98.5 % | (=26.4°) 99.8 % |
| Absorption correction | Semi-empirical | Semi-empirical |
| Ratio Tmin/Tmax | 0.694 | 0.776 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 6428 / 1 / 569 | 5600 / 0 / 300 |
| Goodness-of-fit on F2 | 1.18 | 1.05 |
| Final R indices [I>2sigma(I)] | R1
a = 0.070 wR2b = 0.175 | R1 = 0.021 wR2 = 0.046 |
| R indices (all data) | R1 = 0.072
wR2 = 0.176 | R1 = 0.024 wR2 = 0.047 |
| Absolute structure parameter | 0.00(10) | -0.019(19) |
| Largest diff. peak and hole | 0.76 and -1.24 e.Å-3 | 0.30 and -0.23 e.Å-3 |
| D-H...A | d(D-H) | d(H...A) | d(D...A) | <(DHA) |
| C(2)-H(2B)...Cl(1) | 0.98 | 2.80 | 3.687(8) | 151 |
| C(12)-H(12)...Cl(1)#1 | 0.95 | 2.75 | 3.528(7) | 139 |
| C(23)-H(23)...Cl(1)#1 | 0.95 | 2.63 | 3.562(7) | 166 |
| C(27)-H(27)...Cl(1) | 1.00 | 2.49 | 3.478(7) | 168 |
| C(41)-H(41C)...Cl(1) | 0.98 | 2.98 | 3.944(9) | 167 |
| C(44)-H(44)...Cl(1)#2 | 0.95 | 2.90 | 3.823(7) | 163 |
| C(52)-H(52)...Cl(1) | 0.95 | 2.51 | 3.419(7) | 161 |
| C(1)-H(1)...Cl(2) | 1.00 | 2.54 | 3.499(6) | 161 |
| C(13)-H(13)...Cl(2) | 0.95 | 2.99 | 3.811(7) | 146 |
| C(15)-H(15C)...Cl(2) | 0.98 | 2.96 | 3.909(8) | 162 |
| C(17)-H(17)...Cl(2) | 0.95 | 2.97 | 3.914(7) | 173 |
| C(22)-H(22)...Cl(2) | 0.95 | 2.52 | 3.444(7) | 165 |
| C(28)-H(28B)...Cl(2)#3 | 0.98 | 2.75 | 3.665(8) | 156 |
| C(38)-H(38)...Cl(2) | 0.95 | 2.80 | 3.578(7) | 139 |
| C(51)-H(51)...Cl(2) | 0.95 | 2.65 | 3.540(7) | 156 |
| C(14)-H(14)...Cl(3)#4 | 1.00 | 2.80 | 3.710(7) | 151 |
| C(43)-H(43)...Cl(3)#5 | 0.95 | 2.80 | 3.691(7) | 157 |
| C(48)-H(48)...Cl(3)#5 | 0.95 | 2.66 | 3.595(7) | 168 |
| C(49)-H(49)...Cl(3) | 0.95 | 2.74 | 3.559(8) | 145 |
| O(1)-H(1O)...Cl(3)#4 | 0.97(5) | 2.32(6) | 3.234(6) | 157 |
| O(1)-H(2O)...Cl(3)#6 | 1.02(5) | 2.37(7) | 3.226(6) | 141 |
| C(19)-H(19)...Cl(4)#7 | 0.95 | 2.90 | 3.662(7) | 137 |
| C(35)-H(35)...Cl(4)#4 | 0.95 | 2.99 | 3.593(7) | 122 |
| C(36)-H(36)...Cl(4) | 0.95 | 2.85 | 3.766(8) | 162 |
| C(10)-H(10)...Cl(5)#6 | 0.95 | 2.82 | 3.737(8) | 163 |
| C(45)-H(45)...Cl(5)#8 | 0.95 | 2.86 | 3.608(8) | 136 |
| O(1)-H(2O)...Cl(3)#6 | 1.02(5) | 2.37(7) | 3.226(6) | 141 |
| O(1)-H(1O)...Cl(3)#4 | 0.97(5) | 2.32(6) | 3.234(6) | 157 |
| C(21)-H(21)...O(1) | 0.95 | 2.47 | 3.395(9) | 164 |
| C(25)-H(25)...O(1)#9 | 0.95 | 2.88 | 3.506(9) | 124 |
| C(26)-H(26)...O(1) | 0.95 | 2.75 | 3.672(9) | 164 |
| C(40)-H(40)...O(1)#10 | 1.00 | 2.57 | 3.396(9) | 139 |
| 2R | |
|---|---|
| Cl(1)-Pd(1)-Cl(2) | 90.57(2) |
| Cl(1)-Pd(1)-Cl(3) | 90.39(3) |
| Cl(2)-Pd(1)-Cl(4) | 89.08(2) |
| Cl(3)-Pd(1)-Cl(4) | 90.03(2) |
| D-H...A | d(D-H) | d(H...A) | d(D...A) | <(DHA) |
| C(1)-H(1)...Cl(4)#1 | 1.00 | 2.69 | 3.641(2) | 158 |
| C(3)-H(3)...Cl(2)#2 | 0.95 | 2.55 | 3.463(3) | 160 |
| C(5)-H(5)...Cl(4)#3 | 0.95 | 3.00 | 3.614(3) | 123 |
| C(6)-H(6)...Cl(3)#3 | 0.95 | 2.72 | 3.504(3) | 140 |
| C(7)-H(7)...Cl(2)#1 | 0.95 | 2.70 | 3.486(3) | 140 |
| C(14)-H(14)...Cl(4)#4 | 1.00 | 2.88 | 3.744(3) | 144 |
| C(16)-H(16)...Cl(1)#5 | 0.95 | 2.72 | 3.580(2) | 150 |
| C(16)-H(16)...Cl(3)#5 | 0.95 | 2.73 | 3.374(3) | 126 |
| C(17)-H(17)...Cl(2) | 0.95 | 2.84 | 3.559(3) | 133 |
| C(18)-H(18)...Cl(1) | 0.95 | 2.77 | 3.668(3) | 157 |
| C(25)-H(25)...Cl(4)#3 | 0.95 | 3.00 | 3.648(3) | 126 |
Acknowledgements
References and Notes
- Binnemans, K. Ionic liquid crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef]
- Ding, J.; Armstrong, D. W. Chiral ionic liquids: Synthesis and applications. Chirality 2005, 17, 281–292. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. (Eds.) Ionic Liquid in Synthesis. Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, 2003.
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Comm. 2001, 2399–2407. [Google Scholar] [CrossRef]
- Lin, I. J. B.; Vasam, C. S. Metal-containing ionic liquids and ionic liquid crystals based on imidazolium moiety. J. Organomet. Chem. 2005, 690, 3498–3512. [Google Scholar] [CrossRef]
- Lee, C. K.; Peng, H. H.; Lin, I. J. B. Liquid crystals of N,N '-dialkylimidazolium salts comprising palladium(II) and copper(II) ions. Chem. Mat. 2004, 16, 530–536. [Google Scholar]
- Hasan, M.; Kozhevnikov, I. V.; Siddiqui, M. R. H.; Steiner, A.; Winterton, N. Gold compounds as ionic liquids. Synthesis, structures, and thermal properties of N,N'-dialkylimidazolium tetrachloroaurate salts. Inorg. Chem. 1999, 38, 5637–5641. [Google Scholar]
- Hardacre, C.; Holbrey, J. D.; McCormac, P. B.; McMath, S. E. J.; Nieuwenhuyzen, M.; Seddon, K. R. Crystal and liquid crystalline polymorphism in 1-alkyl-3-methylimidazolium tetrachloropalladate(II) salts. J. Mat. Chem. 2001, 11, 346–350. [Google Scholar] [CrossRef]
- Dyson, P. J. Transition metal chemistry in ionic liquids. Transition Met. Chem. 2002, 27, 353–358. [Google Scholar] [CrossRef]
- Dobbs, W.; Suisse, J.-M.; Douce, L.; Welter, R. Electrodeposition of Silver Particles and Gold Nanoparticles from Ionic Liquid-Crystal Precursors. Angew. Chem. Int. Ed. 2006, 45, 4179–4182. [Google Scholar] [CrossRef]
- Zawartka, W.; Gniewek, A.; Trzeciak, A. M.; Drzewinski, L.; Lis, T. Bis(1-butyl-4-methylpyridinium)tetrachloropalladate(II). Acta Cryst. 2006, E62, M1100–M1102. [Google Scholar]
- Neve, F.; Crispini, A.; Armentano, S.; Francescangeli, O. Synthesis, structure, and thermotropic mesomorphism of layered N-alkylpyridinium tetrahalopalladate(II) salts. Chem. Mat. 1998, 10, 1904–1913. [Google Scholar] [CrossRef]
- Neve, F.; Crispini, A.; Francescangeli, O. Structural studies on layered alkylpyridinium iodopalladate networks. Inorg. Chem. 2000, 39, 1187–1194. [Google Scholar] [CrossRef]
- Neve, F.; Francescangeli, O.; Crispini, A. Crystal architecture and mesophase structure of long-chain N-alkylpyridinium tetrachlorometallates. Inorg. Chim. Acta 2002, 338, 51–58. [Google Scholar] [CrossRef]
- Neve, F.; Francescangeli, O.; Crispini, A.; Charmant, J. A(2) MX4 copper(II) pyridinium salts. From ionic liquids to layered solids to liquid crystals. Chem. Mat. 2001, 13, 2032–2041. [Google Scholar] [CrossRef]
- Taubert, A.; Steiner, P.; Mantion, A. Ionic liquid crystal precursors for inorganic particles: Phase diagram and thermal properties of a CuCl nanoplatelet precursor. J. Phys. Chem. B 2005, 109, 15542–15547. [Google Scholar] [CrossRef]
- Taubert, A.; Arbell, I.; Mecke, A.; Graf, P. Photoreduction of a Crystalline Au(III) Complex: a Solid-state Approach to Metallic Nanostructures. Gold Bull. 2006, 39(4), 205–211. [Google Scholar] [CrossRef]
- Neve, F.; Francescangeli, O. Layered w-Substituted Alkylpyridinium Salts with Inorganic Anions: Effects of H-Bonding Patterns on the Layer Thickness. Cryst. Growth Des. 2005, 5(1), 163–166. [Google Scholar] [CrossRef]
- Genisson, Y.; Marazano, C.; Mehmandoust, M.; Gnecco, D.; Das, B. C. Zincke Reaction with Chiral Primary Amines - a Practical Entry to Pyridinium Salts of Interest in Asymmetric-Synthesis. Synlett 1992, 431–434. [Google Scholar]
- Zincke, T. Ann. Chem. 1903, 330, 361.
- Patrascu, C.; Sugisaki, C.; Mingotaud, C.; Marty, J. D.; Genisson, Y.; Lauth-de Viguerie, N. New pyridinium chiral ionic liquids. Heterocycles 2004, 63, 2033–2041. [Google Scholar] [CrossRef]
- Dullius, J. E. L.; Suarez, P. A. Z.; Einloft, S.; de Souza, R. F.; Dupont, J.; Fischer, J.; De Cian, A. Selective catalytic hydrodimerization of 1,3-butadiene by palladium compounds dissolved in ionic liquids. Organometallics 1998, 17, 815–819. [Google Scholar] [CrossRef]
- Perignon, N.; Marty, J.-D.; Mingotaud, A.-F.; Dumont, M.; Rico-Lattes, I.; Mingotaud, C. Hyperbranched Polymers Analogous to PAMAM Dendrimers for the Formation and Stabilization of Gold Nanoparticles. Macromolecules 2007, 40, 3034–3041. [Google Scholar] [CrossRef]
- Gascon, I.; Marty, J.-D.; Gharsa, T.; and Mingotaud, C. Hyperbranched Polymers Analogous to PAMAM Formation of gold nanoparticles in a side-chain liquid crystalline network: Influence of the structure and macroscopic order of the material. Chem. Mat. 2005, 17, 5228–5230. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 1R, 1S, 2R, 2S are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Tourneux, E.; Gornitzka, H.; Marty, J.-D.; Viguerie, N.L.-d. Palladate Salts from Chiral Pyridinium Ionic Liquids: Synthesis and Crystal Structures. Molecules 2007, 12, 1940-1949. https://doi.org/10.3390/12081940
Tourneux E, Gornitzka H, Marty J-D, Viguerie NL-d. Palladate Salts from Chiral Pyridinium Ionic Liquids: Synthesis and Crystal Structures. Molecules. 2007; 12(8):1940-1949. https://doi.org/10.3390/12081940
Chicago/Turabian StyleTourneux, Emmanuel, Heinz Gornitzka, Jean-Daniel Marty, and Nancy Lauth-de Viguerie. 2007. "Palladate Salts from Chiral Pyridinium Ionic Liquids: Synthesis and Crystal Structures" Molecules 12, no. 8: 1940-1949. https://doi.org/10.3390/12081940




