Trehalose and Trehalose-based Polymers for Environmentally Benign, Biocompatible and Bioactive Materials
Abstract
:Introduction
Structure, Properties, and Applications of Trehalose
Structure and properties
- 4% Aqueous trehalose solutions resist degradation between pH 3.5 and 10, at 100°C (24 h);
- The sugar is unreactive with amines, amino acids, and proteins (Maillard reaction); and
- Unlike sucrose, trehalose resists caramelization (browning) in prepared foods, and can be used as an excellent food bulking agent.
| Melting point | dihydrate | 97.0°C |
| anhydride | 210.5°C | |
| Heat of fusion | dihydrate | 57.8 kJ mol-1 |
| anhydride | 53.4 kJ mol-1 | |
| Solubility | 68.9 g/100 g H2O at 20°C | |
| Optical rotation | [α]d +178° | |
| Relative sweetness | 45% of sucrose | |
| Digestibility | digested and absorbed by the small intestine | |
| pH stability of solution | > 99% (pH 3.5-10, at 100°C for 24 h) | |
| Heat stability of solution | > 99% (at 120°C for 90 min) | |
Mechanisms of phospholipid and protein stabilization by trehalose

Protection of proteins by trehalose
- (a)
- The direct interaction between trehalose molecules and proteins through hydrogen bonds (water replacement hypothesis);
- (b)
- The trapping of water molecules close to protein surface (water-layer hypothesis); and
- (c)
- The entrapment of proteins conformations in high viscosity trehalose glasses (mechanical-entrapment hypothesis) [91].
Protection of mammalian cells by trehalose
Organ preservation and applications in diagnostic medicine
Uses of trehalose in functional foods, flowers, cosmetics, and other applications
Production of Trehalose
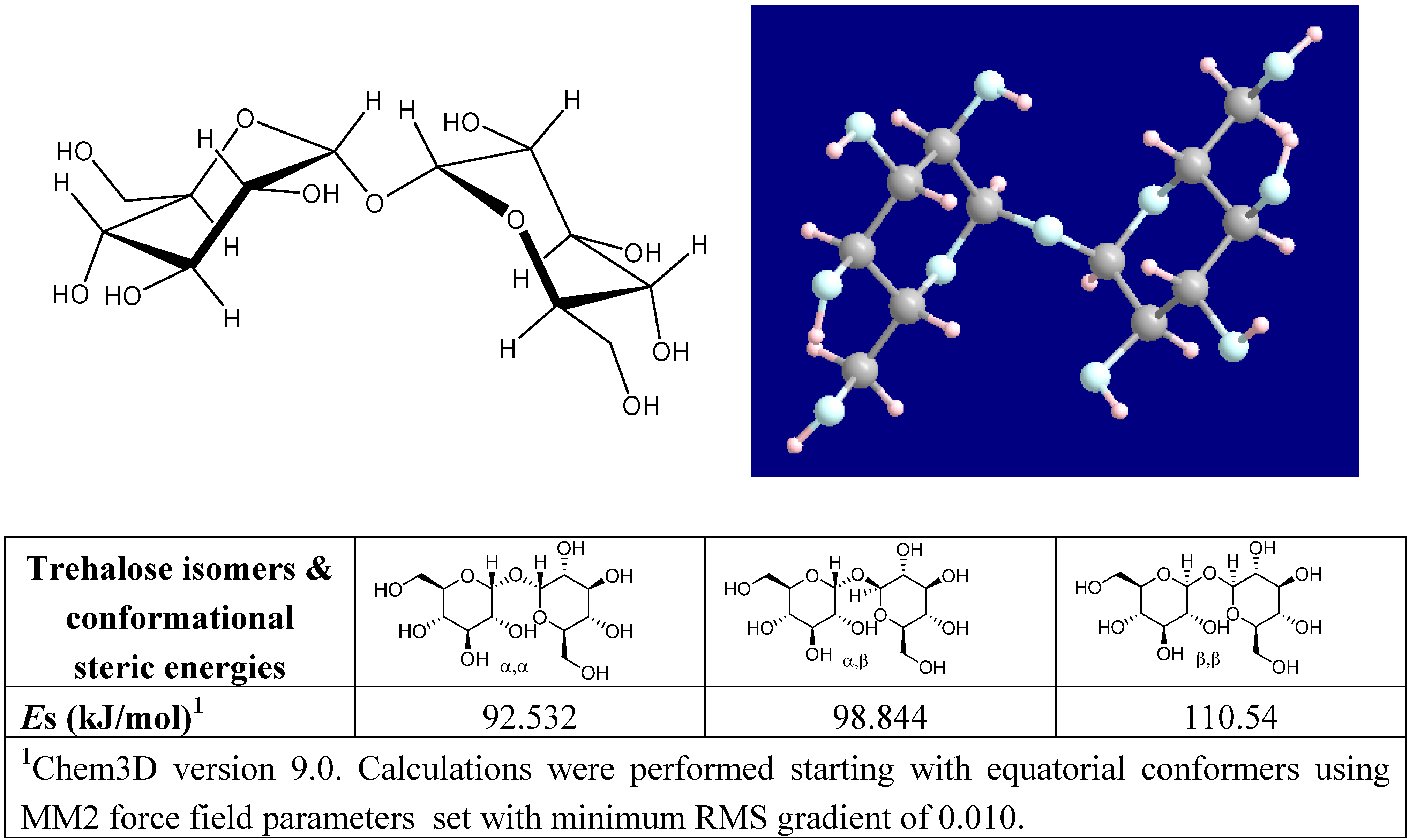
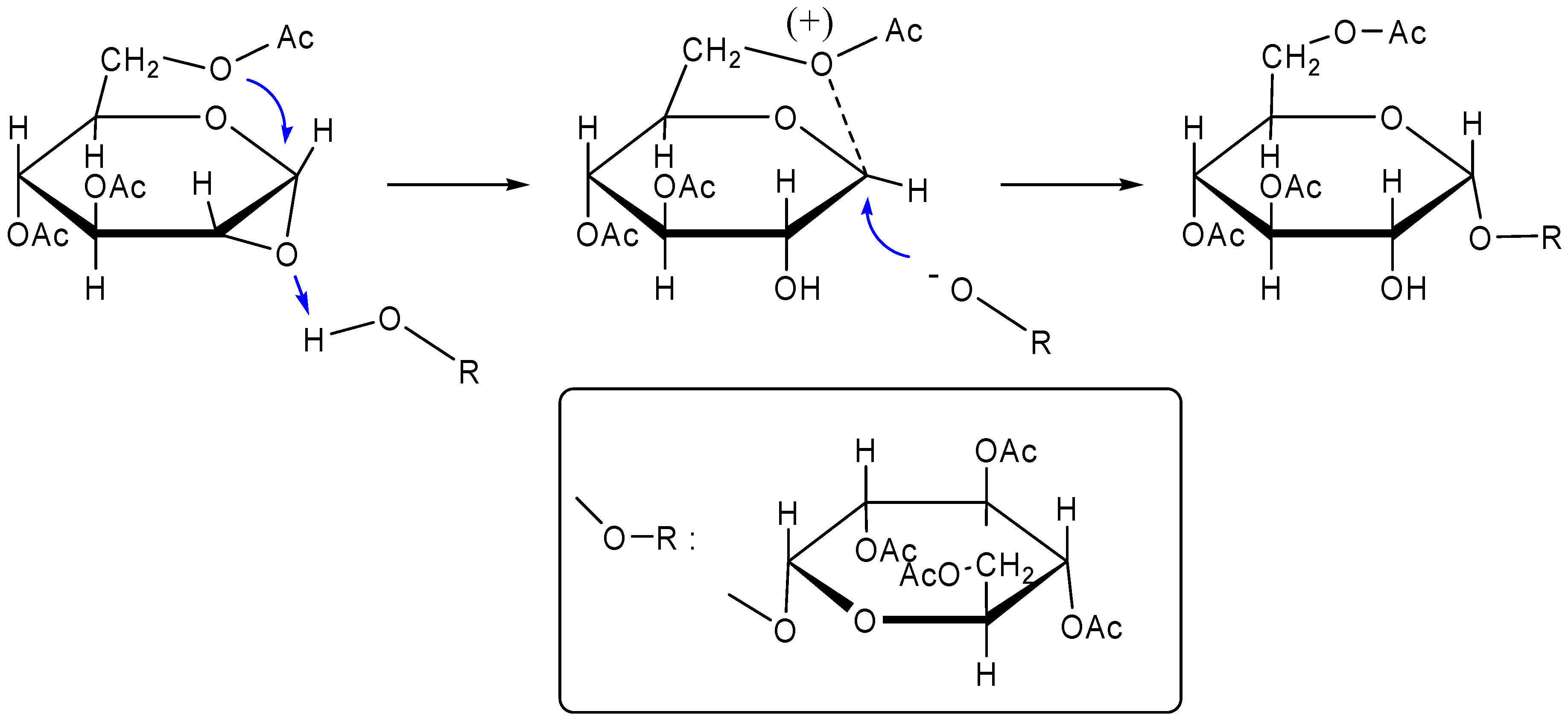
Chemical synthesis
Biosynthesis
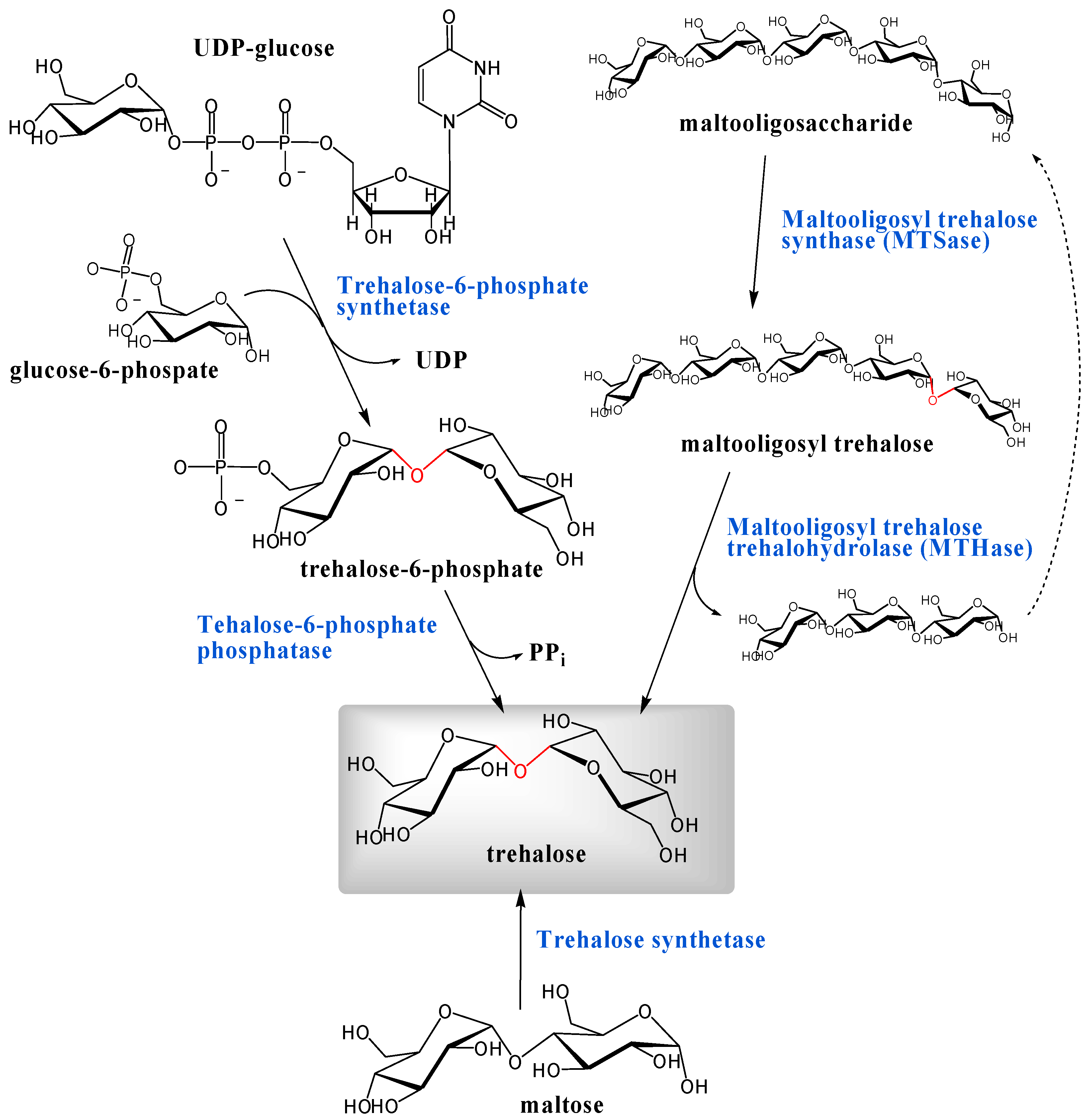
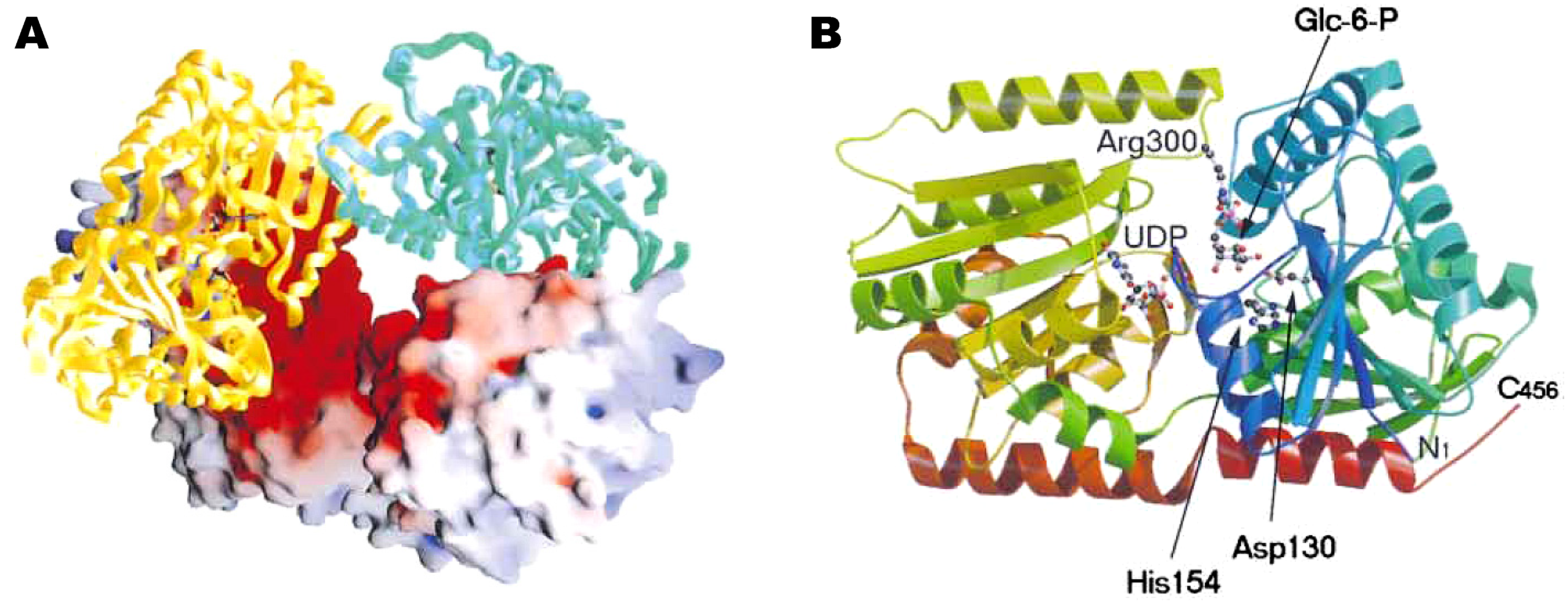

Industrial production
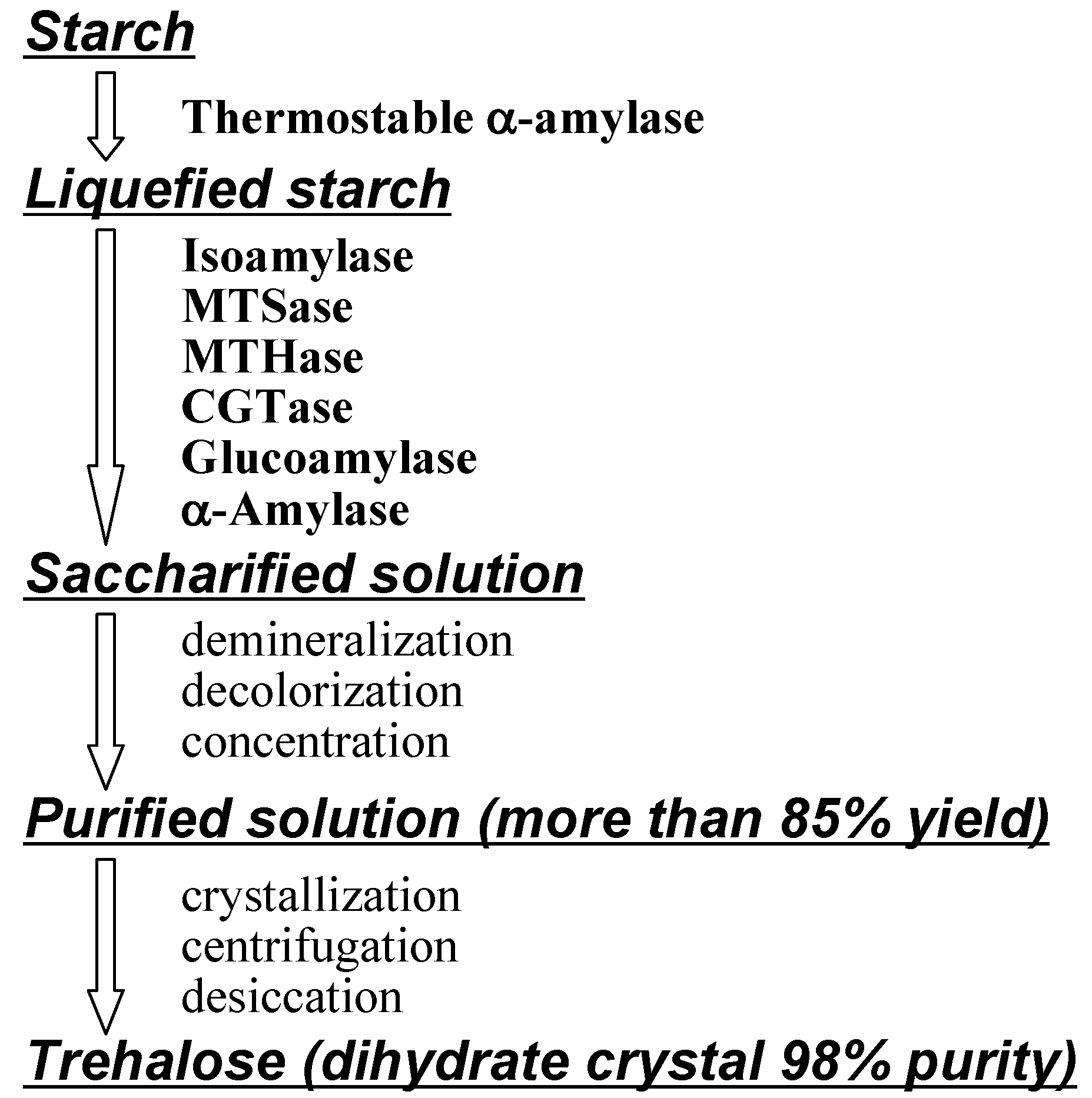
Trehalose-based Polymers
Network polymers
- (a)
- The price being very high (> $200/kg) until 1995 (now < $3/kg);
- (b)
- It has no anomeric hydroxyl group whose reactivity is different from other hydroxyl groups; and
- (c)
- The disaccharide is devoid of a galactosyl residue that shows affinity for interaction with cell receptors.

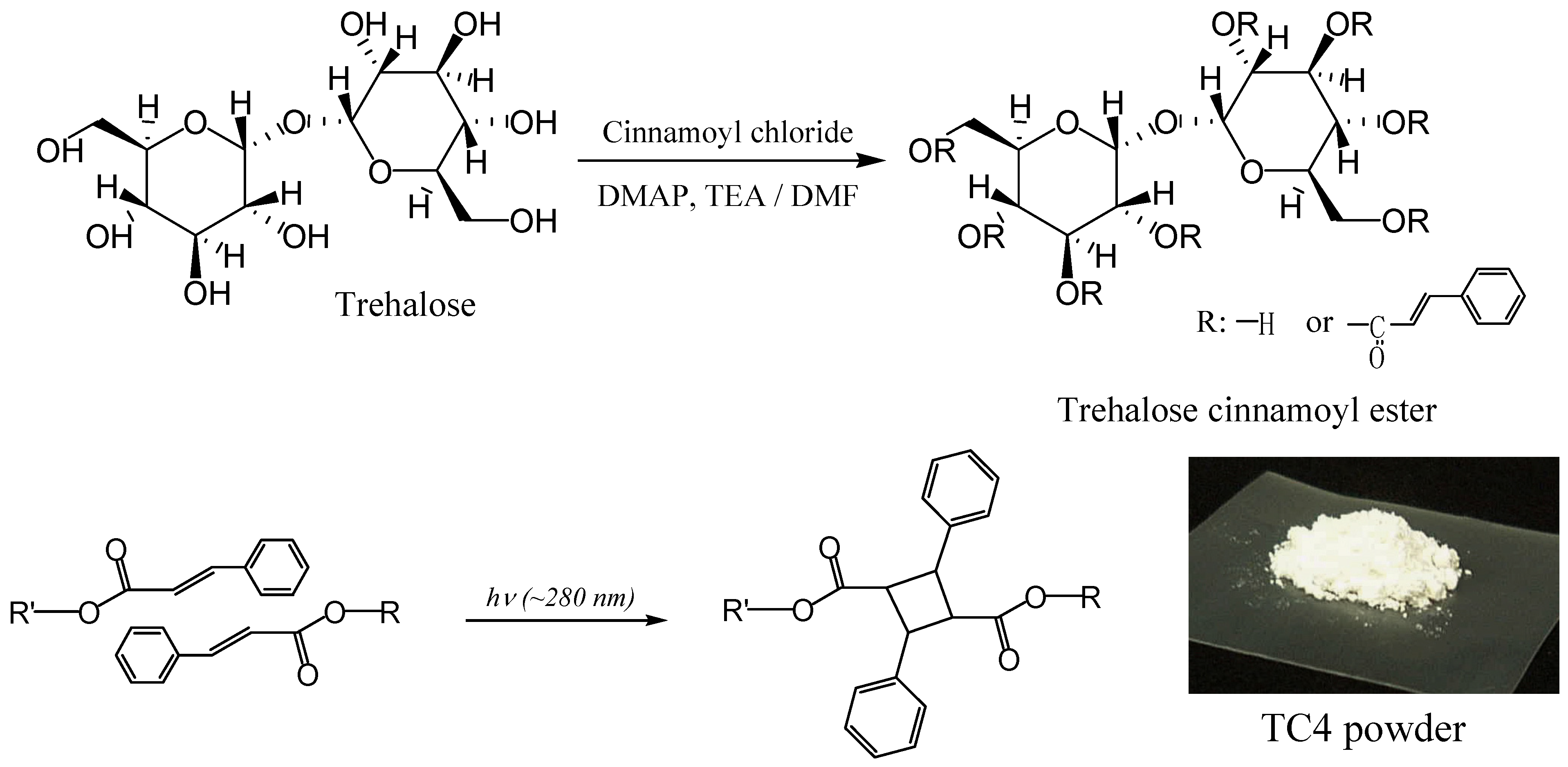

Linear Polymers
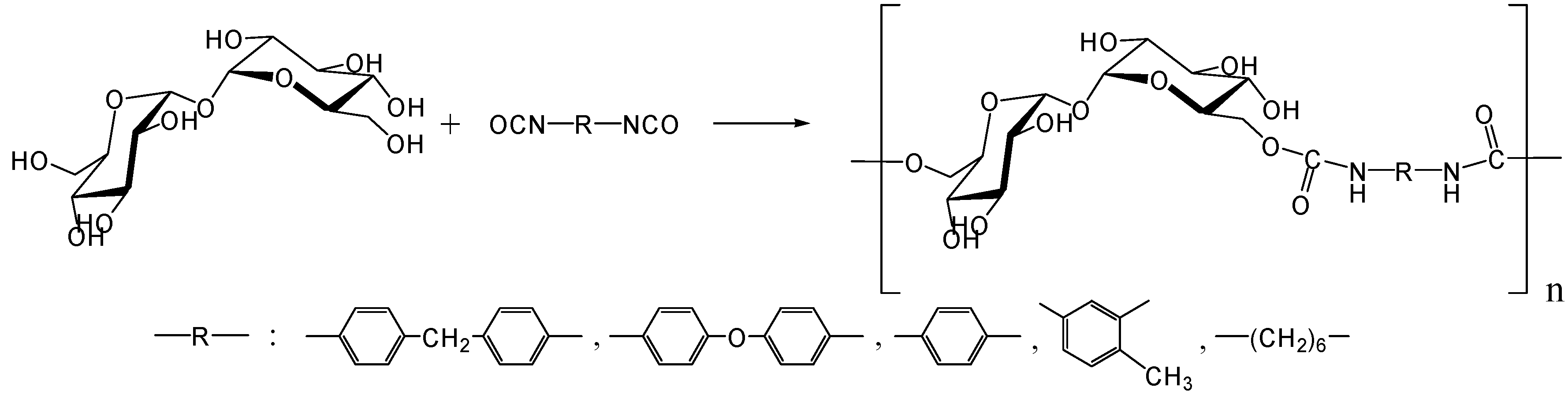
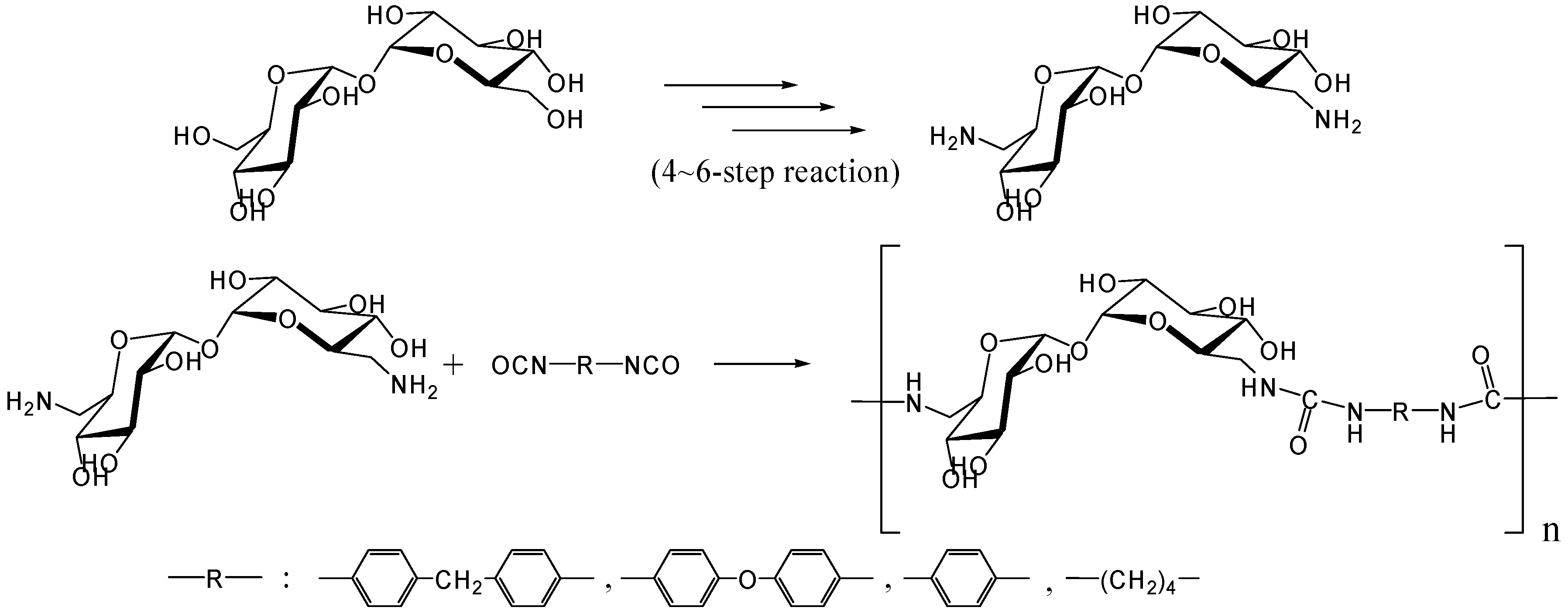
Polyaddition with diisocyanates
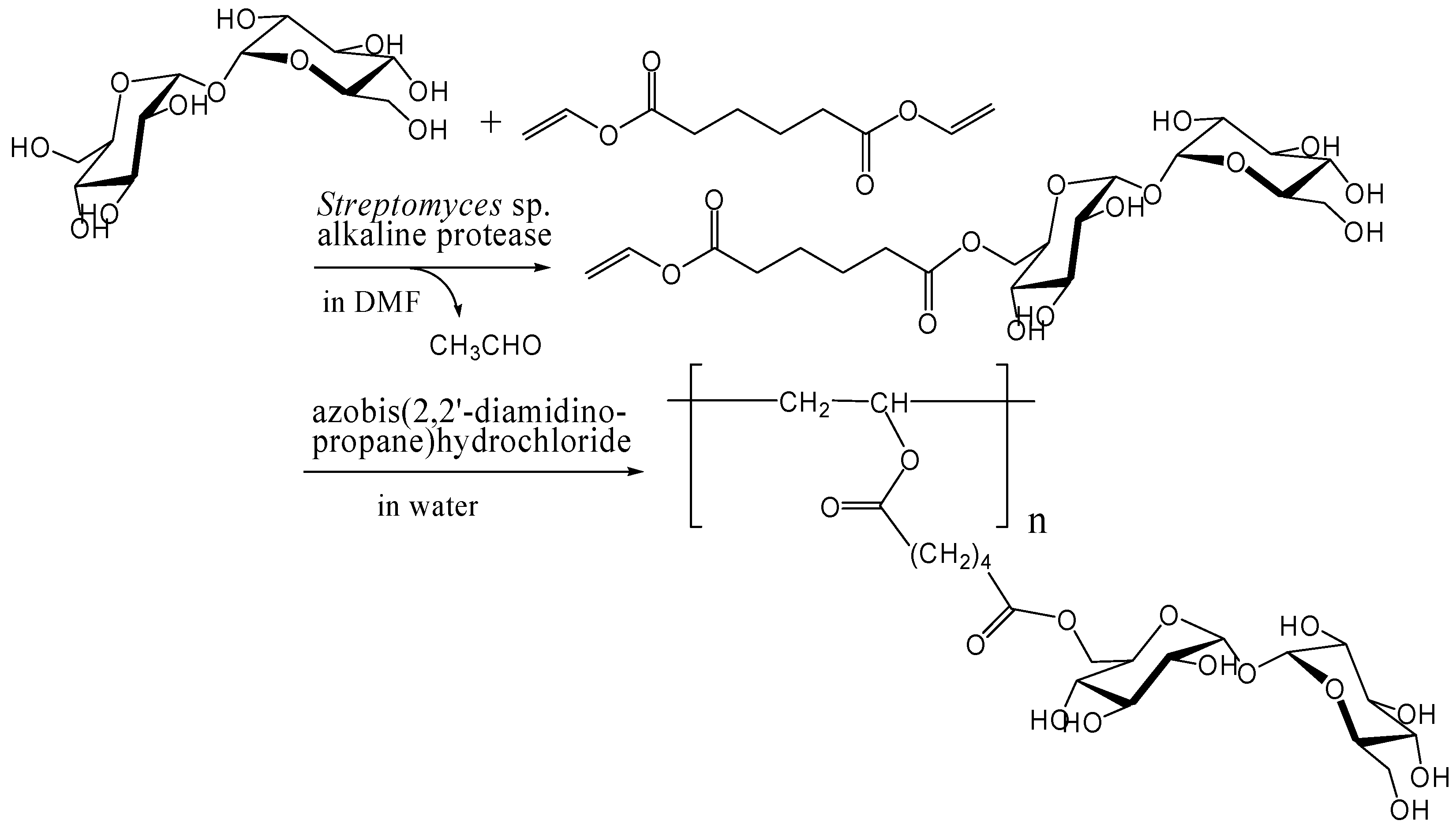
Enzymatic and chemoenzymatic synthesis
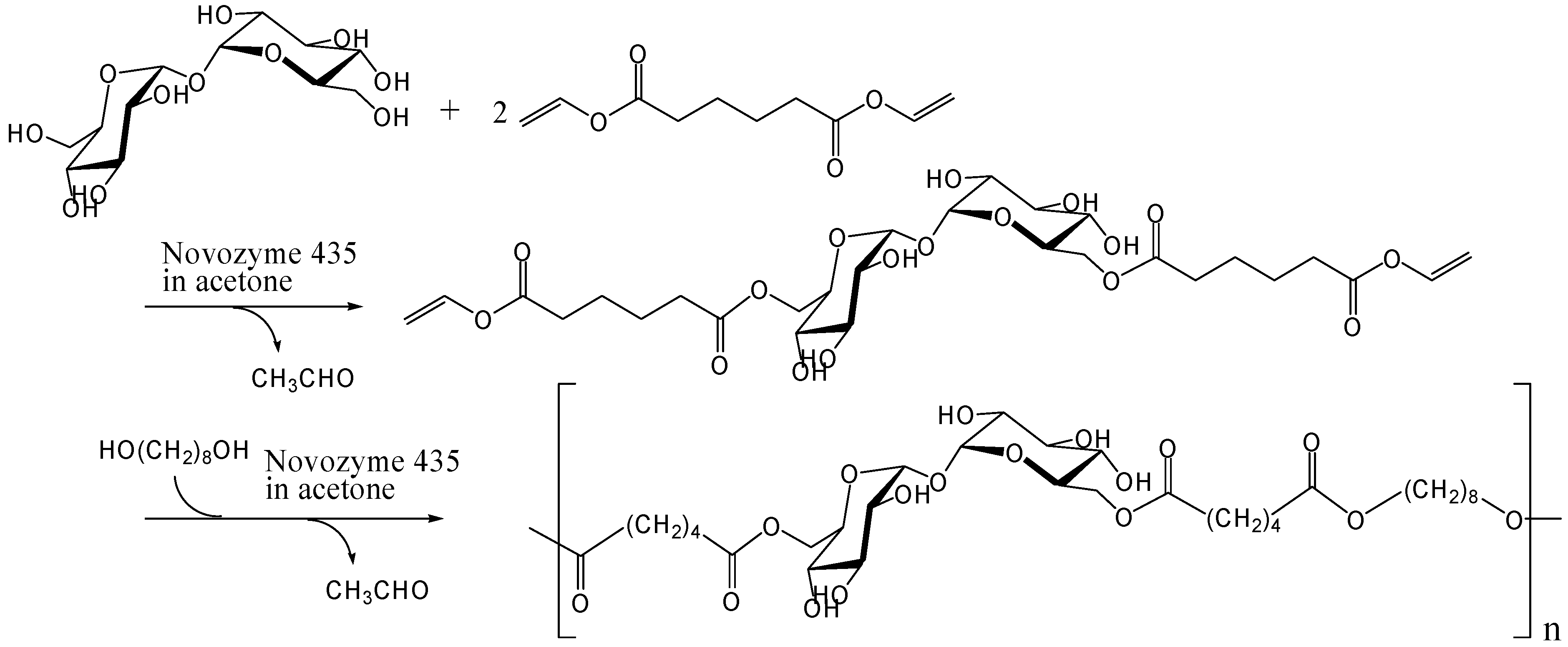
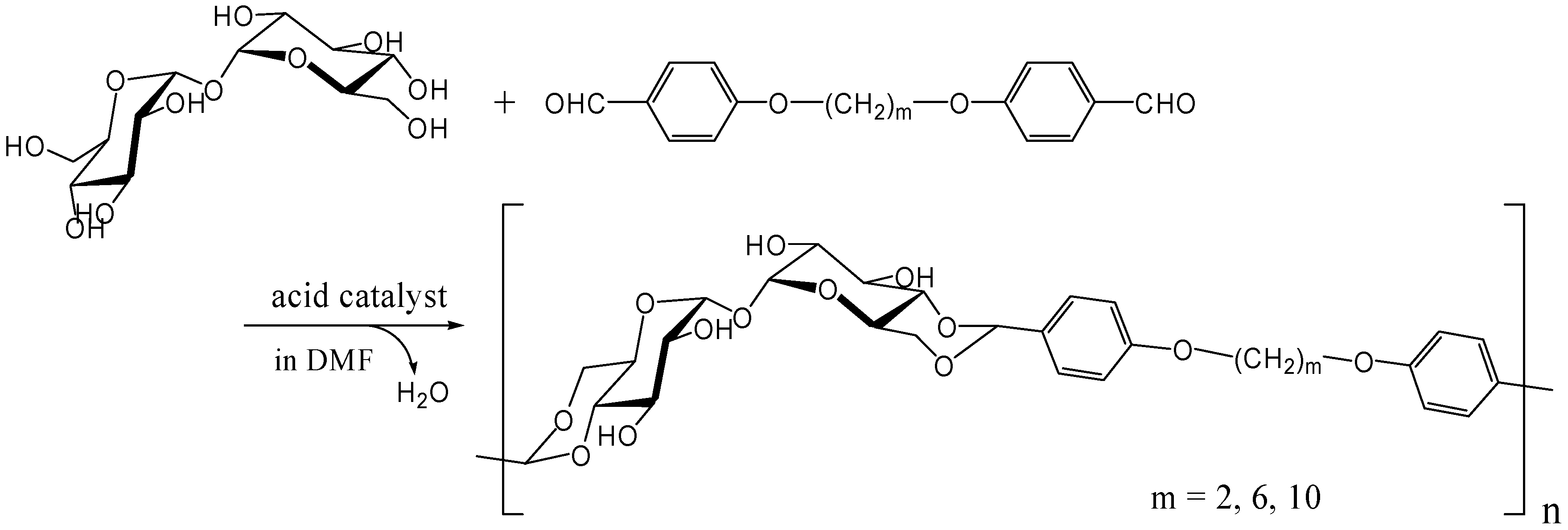
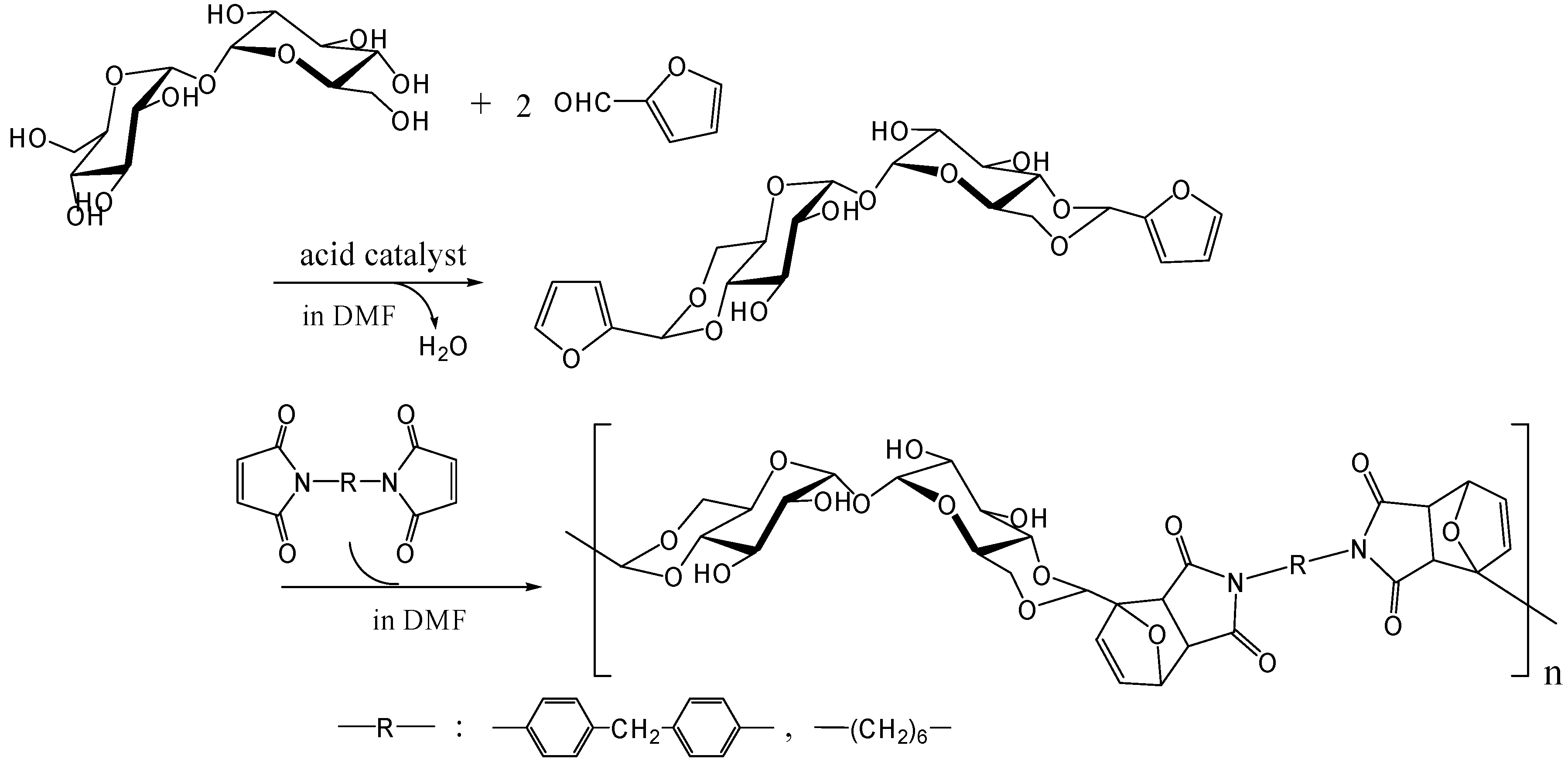
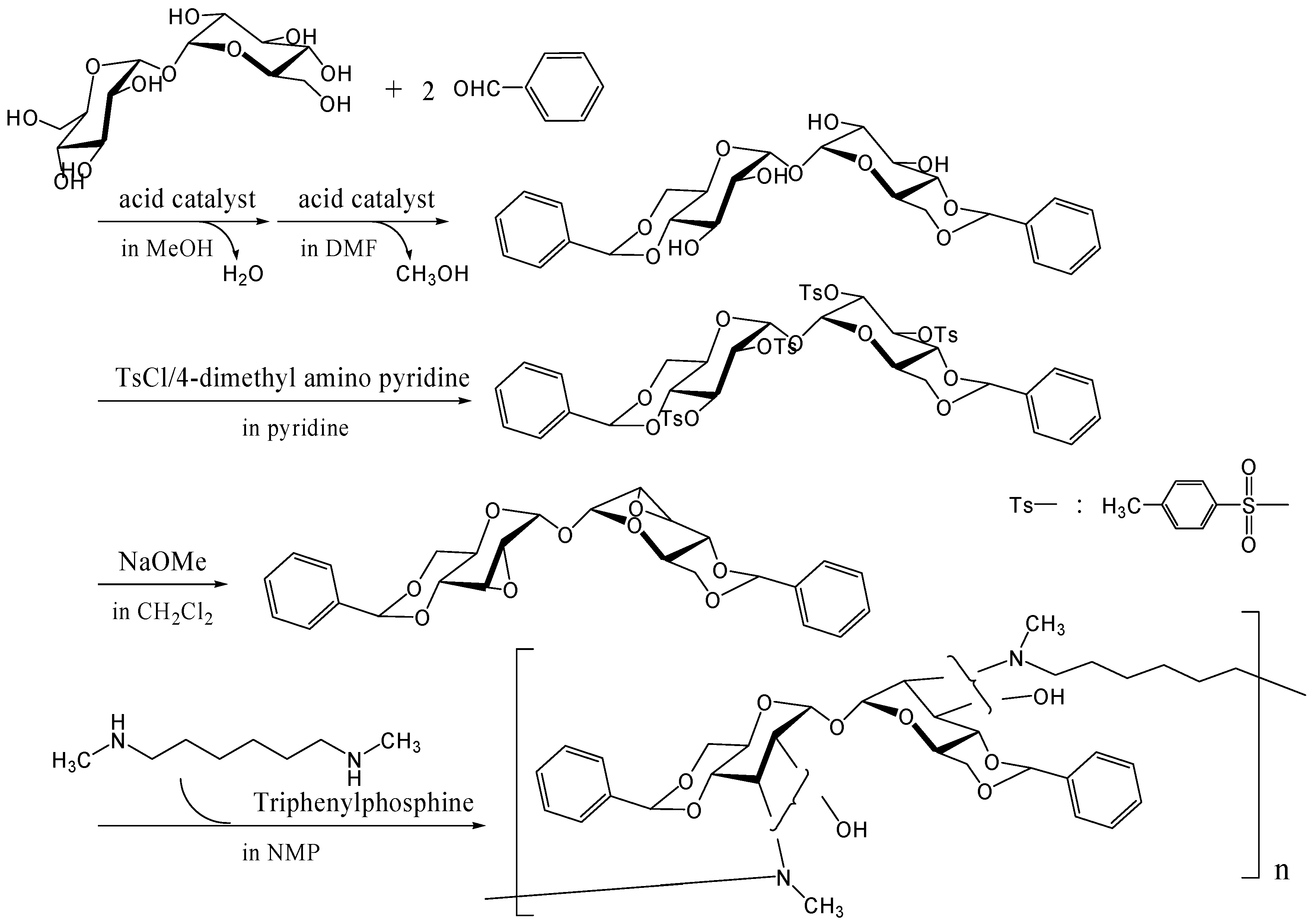
Acetalization with dialdehydes

Hydrosilylation

Diels-Alder polymerization
Azide-alkyne Huisgen cycloaddition (click reaction)
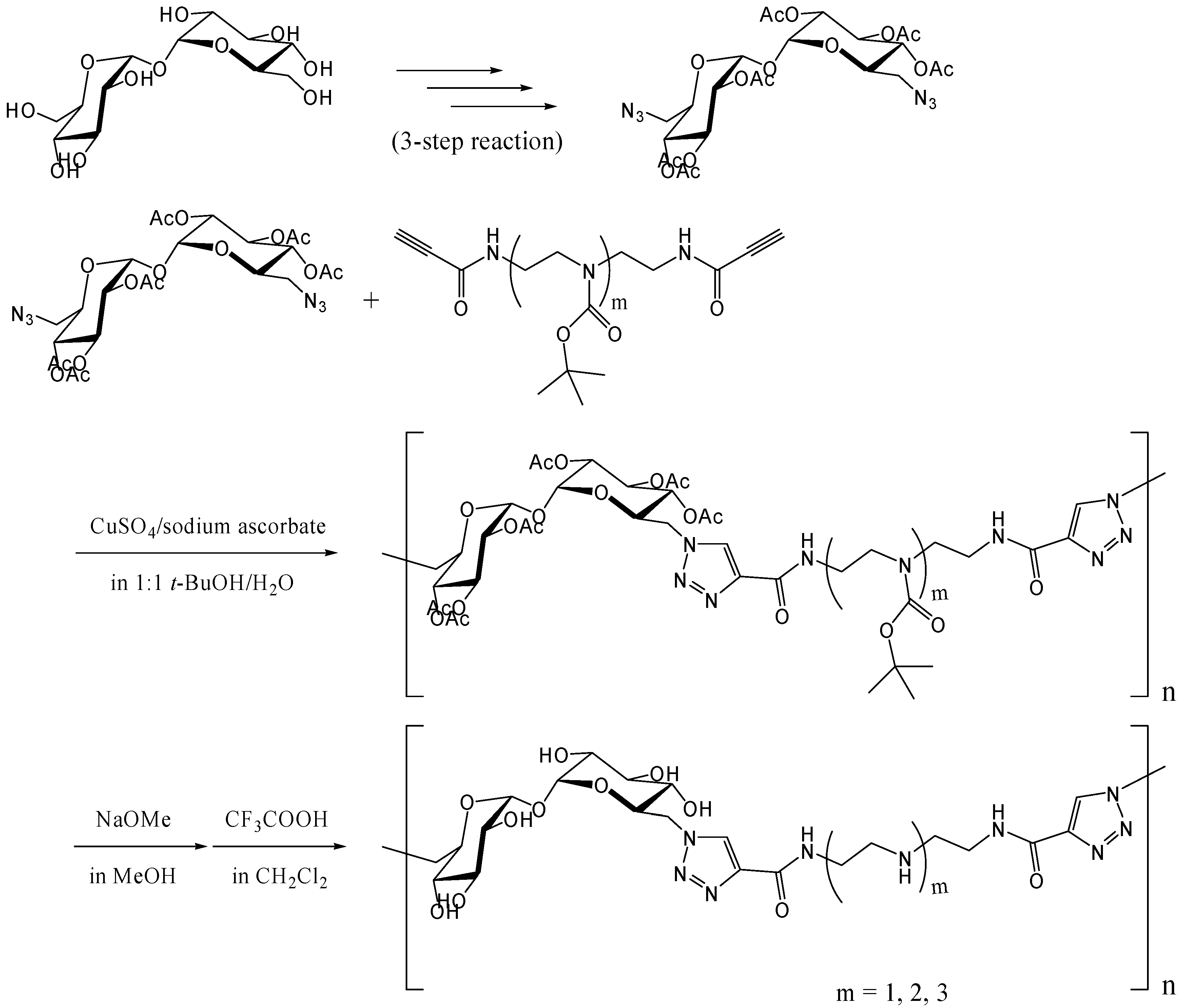
Other methods
Conclusions
Acknowledgements
References and Notes
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: a multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Berthelot, M. Sur le trehalose, nouvelle espece de sucre. Compt. Rend. Hebd. Seanc. Acad. Sci., Paris 1858, 46, 1276–1279. [Google Scholar]
- Elbein, A.D. The metabolism of α,α-trehalose. Adv. Carbohydr. Chem. Biochem. 1974, 30, 227–256. [Google Scholar] [CrossRef]
- Wyatt, G.R.; Kalf, G.F. The chemistry of insect hemolymph: II. Trehalose and other carbohydrates. J. Gen. Physiol. 1957, 40, 833–847. [Google Scholar] [CrossRef]
- Bucher, T.; Klingenberg, M. Wege des Wasserstoffs in der lebendigen Organisation. Angew. Chem. 1958, 70, 552–570. [Google Scholar] [CrossRef]
- Evans, D.R.; Dethier, V.G. The regulation of taste thresholds for sugars in the blowfly. J. Insect Physiol. 1957, 1, 3–17. [Google Scholar] [CrossRef]
- Howden, G.F.; Kilby, B.A. Trehalose and trehalase in the locust. Chem. Ind.(Lond.) 1956, 1453–1454. [Google Scholar]
- Bourquelot, M.E.M. Remarques sur les ferments solubles secretes par l'Aspergillus niger et le Pencillium. Compt. Rend. Soc. Biol. IX 1893, 5, 653. [Google Scholar]
- Paiva, C.L.; Panek, A.D. Biotechnological applications of the disaccharide trehalose. Biotechnol. Annu. Rev. 1996, 2, 293–314. [Google Scholar] [CrossRef]
- Hey, A.E.; Elbein, A.D. Partial purification and properties of a trehalase from Streptomyces hygroscopicus. J. Bacteriol. 1968, 96, 105–110. [Google Scholar]
- Dahlqvist, A.; Brun, A. A method for the histochemical demonstration of disaccharidase activities: application to invertase and trehalase in some animal tissues. J. Histochem. Cytochem. 1962, 10, 294–302. [Google Scholar] [CrossRef]
- Sacktor, B. Trehalase and the transport of glucose in the mammalian kidney and intestine. Proc. Natl. Acad. Sci. USA 1968, 60, 1007–1014. [Google Scholar] [CrossRef]
- Sasai-Takedatsu, M.; Taketani, S.; Nagata, N.; Furukawa, T.; Tokunaga, R.; Kojima, T.; Kobayashi, Y. Human trehalase: characterization, localization, and its increase in urine by renal proximal tubular damage. Nephron 1996, 73, 179–185. [Google Scholar] [CrossRef]
- Ishihara, R.; Taketani, S.; Sasai-Takedatsu, M.; Kino, M.; Tokunaga, R.; Kobayashi, Y. Molecular cloning, sequencing and expression of cDNA encoding human trehalase. Gene 1997, 202, 69–74. [Google Scholar] [CrossRef]
- Ishihara, R.; Taketani, S.; Sasai-Takedatsu, M.; Adachi, Y.; Kino, M.; Furuya, A.; Hanai, N.; Tokunaga, R.; Kobayashi, Y. ELISA for urinary trehalase with monoclonal antibodies: a technique for assessment of renal tubular damage. Clin. Chem. 2000, 46, 636–643. [Google Scholar]
- Maestracci, D. Enzymic solubilization of the human intestinal brush border membrane enzymes. Biochim. Biophys. Acta. 1976, 433, 469–481. [Google Scholar] [CrossRef]
- Gudmand-Høyer, E.; Skovbjerg, H. Disaccharide digestion and maldigestion. Scand. J. Gastroenterol. 1996, 31 (Suppl.), 111–121. [Google Scholar] [CrossRef]
- Trevelyan, W.E.; Harrison, J.S. Studies on yeast metabolism. 5. The trehalose content of baker's yeast during anaerobic fermentation. Biochem. J. 1956, 62, 177–183. [Google Scholar]
- Nwaka, S.; Holzer, H. Molecular biology of trehalose and the trehalases in the yeast Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 1998, 58, 197–237. [Google Scholar] [CrossRef]
- Clegg, J.S.; Filosa, M.F. Trehalose in the cellular slime mold, Dictyostelium mucoroides. Nature 1961, 192, 1077–1078. [Google Scholar] [CrossRef]
- Sussman, A.S.; Lingappa, B.T. Role of trehalose in ascospores of Neurospora tetrasperma. Science 1959, 130, 1343. [Google Scholar]
- Giots, F.; Donaton, M.C.; Thevelein, J.M. Inorganic phosphate is sensed by specific phosphate carriers and acts in concert with glucose as a nutrient signal for activation of the protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 2003, 47, 1163–1181. [Google Scholar] [CrossRef]
- Argüelles, J.C.; Rodriguez, T.; Alvarez-Peral, F.J. Trehalose hydrolysis is not required for human serum-induced dimorphic transition in Candida albicans: evidence from a tps1/tps1 mutant deficient in trehalose synthesis. Res. Microbiol. 1999, 150, 521–529. [Google Scholar] [CrossRef]
- Argüelles, J.C. Physiological roles of trehalose in bacteria and yeasts: a comparative analysis. Arch. Microbiol. 2000, 174, 217–224. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science 1984, 223, 701–703. [Google Scholar]
- Keilin, D. The Leeuwenhoek Lecture: The Problem of Anabiosis or Latent Life: History and Current Concept. Proc. R. Soc. London. B 1959, 150, 149–191. [Google Scholar] [CrossRef]
- Clegg, J.S. Cryptobiosis - a peculiar state of biological organization. Comp. Biochem. Physiol. B 2001, 128, 613–624. [Google Scholar] [CrossRef]
- Neuman, Y. Cryptobiosis: A new theoretical perspective. Prog. Biophys. Mol. Biol. 2006, 92, 258–267. [Google Scholar] [CrossRef]
- Tunnacliffe, A.; Lapinski, J. Resurrecting Van Leeuwenhoek's rotifers: a reappraisal of the role of disaccharides in anhydrobiosis. Phil. Trans. R. Soc. B: Biol. Sci. 2003, 358, 1755–1771. [Google Scholar] [CrossRef]
- Clegg, J.S. Metabolic studies of cryptobiosis in encysted embryos of Artemia salina. Comp. Biochem. Physiol. 1967, 20, 801–809. [Google Scholar] [CrossRef]
- Guidetti, R.; Jönsson, K.I. Long-term anhydrobiotic survival in semi-terrestrial micrometazoans. J. Zool. 2002, 257, 181–187. [Google Scholar] [CrossRef]
- Rebecchi, L.; Altiero, T.; Guidetti, R. Anhydrobiosis: the extreme limit of desiccation tolerance. Invertebr. Surv. J. 2007, 4, 65–81. [Google Scholar]
- Wright, J.C. Cryptobiosis 300 Years on from van Leuwenhoek: What have we learned about tardigrades? Zool. Anzeiger 2001, 240, 563–582. [Google Scholar] [CrossRef]
- Jönsson, K.I.; Bertolani, R. Facts and fiction about long-term survival in tardigrades. J. Zool. 2001, 255, 121–123. [Google Scholar] [CrossRef]
- Rebecchi, L.; Guidetti, R.; Borsari, S.; Altiero, T.; Bertolani, R. Dynamics of long-term anhydrobiotic survival of lichen-dwelling tardigrades. Hydrobiologia 2006, 558, 23–30. [Google Scholar] [CrossRef]
- Ramløv, H.; Westh, P. Survival of the cryptobiotic eutardigrade Adorybiotus coronifer during cooling to -196°C: Effect of cooling rate, trehalose level, and short-term acclimation. Cryobiology 1992, 29, 125–130. [Google Scholar] [CrossRef]
- Clegg, J.S. Free glycerol in dormant cysts of the brine shrimp Artemia salina, and its disappearance during development. Biol. Bull. 1962, 123, 295–301. [Google Scholar] [CrossRef]
- Clegg, J.S. The origin of threhalose and its significance during the formation of encysted dormant embryos of Artemia salina. Comp. Biochem. Physiol. 1965, 14, 135–143. [Google Scholar] [CrossRef]
- Clegg, J.S. Hydration-dependent metabolic transitions and the state of cellular water in Artemia cysts. In Dry Biological Systems; Crowe, J.H., Clegg, J.S., Eds.; Academic Press: New York, 1978; pp. 117–153. [Google Scholar]
- Clegg, J.S. The physical properties and metabolic status of Artemia cysts at low water contents: the water replacement hypothesis. In Membranes, Metabolism and Dry Organisms; Leopold, A.C., Ed.; Cornell University Press: New York, 1986; pp. 169–187. [Google Scholar]
- Madin, K.A.C.; Crowe, J.H. Anhydrobiosis in nematodes: Carbohydrate and lipid metabolism during dehydration. J. Exp. Zool. 1975, 193, 335–342. [Google Scholar] [CrossRef]
- Higa, L.M.; Womersley, C.Z. New insights into the anhydrobiotic phenomenon: The effects of trehalose content and differential rates of evaporative water loss on the survival of Aphelenchus avenae. J. Exp. Zool. 1993, 267, 120–129. [Google Scholar] [CrossRef]
- Behm, C.A. The role of trehalose in the physiology of nematodes. Int. J. Parasitol. 1997, 27, 215–229. [Google Scholar] [CrossRef]
- Crowe, J.H. The physiology of cryptobiosis in tardigrades. Mem. Ist. Ital. Idrobiol. 1975, 32 (Suppl.), 37–59. [Google Scholar]
- Crowe, J.H. Anhydrobiosis: An unsolved problem. Amer. Naturalist 1971, 105, 563–573. [Google Scholar]
- Crowe, J.H.; Madin, K.A. Anhydrobiosis in tardigrades and nematodes. Trans. Am. Microsc. Soc. 1974, 93, 513–524. [Google Scholar] [CrossRef]
- Westh, P.; Ramløv, H. Trehalose accumulation in the tardigrade Adorybiotus coronifer during anhydrobiosis. J. Exp. Zool. 1991, 258, 303–311. [Google Scholar] [CrossRef]
- Asahina, E.; Tanno, K. A large amount of trehalose in a frost-resistant insect. Nature 1964, 204, 1222–1222. [Google Scholar] [CrossRef]
- Diniz-Mendes, L.; Bernardes, E.; de Araujo, P. S.; Panek, A. D.; Paschoalin, V. M. F. Preservation of frozen yeast cells by trehalose. Biotechnol. Bioeng. 1999, 65, 572–578. [Google Scholar] [CrossRef]
- Alamillo, J.; Almoguera, C.; Bartels, D.; Jordano, J. Constitutive expression of small heat shock proteins in vegetative tissues of the resurrection plant Craterostigma plantagineum. Plant Mol. Biol. 1995, 29, 1093–1099. [Google Scholar] [CrossRef]
- Clegg, J.S.; Jackson, S.A.; Warner, A.H. Extensive intracellular translocations of a major protein accompany anoxia in embryos of Artemia franciscana. Exp. Cell Res. 1994, 212, 77–83. [Google Scholar] [CrossRef]
- Clegg, J.S.; Willsie, J.K.; Jackson, S.A. Adaptive significance of a small heat shock/α-crystallin protein (p26) in encysted embryos of the brine shrimp, Artemia franciscana. Amer. Zool. 1999, 39, 836–847. [Google Scholar]
- Clegg, J.S. Desiccation tolerance in encysted embryos of the animal extremophile, Artemia. Integr. Comp. Biol. 2005, 45, 715–724. [Google Scholar] [CrossRef]
- Katoh, H.; Asthana, R.K.; Ohmori, M. Gene expression in the cyanobacterium Anabaena sp. PCC7120 under desiccation. Microbial Ecol. 2004, 47, 164–174. [Google Scholar] [CrossRef]
- Tanaka, M.; Earl, A. M.; Howell, H. A.; Park, M.-J.; Eisen, J. A.; Peterson, S. N.; Battista, J. R. Analysis of Deinococcus radiodurans's transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics 2004, 168, 21–33. [Google Scholar]
- Ramløv, H.; Westh, P. Cryptobiosis in the eutardigrade Adorybiotus (Richtersius) coronifer: Tolerance to alcohols, temperature and de novo protein synthesis. Zool. Anzeiger 2001, 240, 517–523. [Google Scholar] [CrossRef]
- Schill, R.O.; Steinbruck, G.H.B.; Kohler, H.-R. Stress gene (hsp70) sequences and quantitative expression in Milnesium tardigradum (Tardigrada) during active and cryptobiotic stages. J. Exp. Biol. 2004, 207, 1607–1613. [Google Scholar] [CrossRef]
- Jönsson, K.I.; Schill, R.O. Induction of Hsp70 by desiccation, ionising radiation and heat-shock in the eutardigrade Richtersius coronifer. Comp. Biochem. Physiol. B 2007, 146, 456–460. [Google Scholar] [CrossRef]
- Hudson, C.S. Some numerical relations among the rotatory powers of the compound sugars. J. Am. Chem. Soc. 1916, 38, 1566–1575. [Google Scholar] [CrossRef]
- Taga, T.; Senma, M.; Osaki, K. The crystal and molecular structure of trehalose dihydrate. Acta Crystallogr. 1972, B28, 3258–3263. [Google Scholar] [CrossRef]
- Roser, B. Trehalose, a new approach to premium dried foods. Trends Food Sci. Technol. 1991, 2, 166–169. [Google Scholar] [CrossRef]
- Higashiyama, T. Novel functions and applications of trehalose. Pure Appl. Chem. 2002, 74, 1263–1269. [Google Scholar] [CrossRef]
- Clegg, J.S.; Seitz, P.; Seitz, W.; Hazlewood, C.F. Cellular responses to extreme water loss: The water-replacement hypothesis. Cryobiology 1982, 19, 306–316. [Google Scholar]
- Webb, S.J. Bound Water in Biological Integrity; Charles C. Thomas Publications: Springfield, 1965. [Google Scholar]
- Warner, D.T. Some possible relationships of carbohydrates and other biological components with the water structure at 37°C. Nature 1962, 196, 1055–1058. [Google Scholar] [CrossRef]
- Seitz, P.K.; Chang, D.C.; Hazlewood, C.F.; Rorschach, H.E.; Clegg, J.S. The self-diffusion of water in Artemia cysts. Arch. Biochem. Biophys. 1981, 210, 517–524. [Google Scholar] [CrossRef]
- Crowe, L.M.; Mouradian, R.; Crowe, J.H.; Jackson, S.A.; Womersley, C. Effects of carbohydrates on membrane stability at low water activities. Biochim. Biophys. Acta 1984, 769, 141–150. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Infrared spectroscopic studies on interactions of water and carbohydrates with a biological membrane. Arch. Biochem. Biophys. 1984, 232, 400–407. [Google Scholar] [CrossRef]
- Crowe, L.M.; Crowe, J.H.; Rudolph, A.; Womersley, C.; Appel, L. Preservation of freeze-dried liposomes by trehalose. Arch. Biochem. Biophys. 1985, 242, 240–247. [Google Scholar] [CrossRef]
- Crowe, L.M.; Womersley, C.; Crowe, J.H.; Reid, D.; Appel, L.; Rudolph, A. Prevention of fusion and leakage in freeze-dried liposomes by carbohydrates. Biochim. Biophys. Acta 1986, 861, 131–140. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Carpenter, J.F.; Wistrom, C.A. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem. J. 1987, 242, 1–10. [Google Scholar]
- Crowe, L.M. Lessons from nature: the role of sugars in anhydrobiosis. Comp. Biochem. Physiol. A 2002, 131, 505–513. [Google Scholar] [CrossRef]
- Lambruschini, C.; Relini, A.; Ridi, A.; Cordone, L.; Gliozzi, A. Trehalose interacts with phospholipid polar heads in langmuir monolayers. Langmuir 2000, 16, 5467–5470. [Google Scholar] [CrossRef]
- Chandrasekhar, I.; Gaber, B.P. Stabilization of the bio-membrane by small molecules: interaction of trehalose with the phospholipid bilayer. J. Biomol. Struct. Dyn. 1988, 5, 1163–71. [Google Scholar] [CrossRef]
- Burke, M.J. The vitreous state and survival of anhydrous biological systems. In Membranes, metabolism and dry organisms; Cornell University Press: New York, 1986; pp. 358–364. [Google Scholar]
- Franks, F. Biophysics and Biochemistry at Low Temperatures; Cambridge University Press: Cambridge, U.K., 1985. [Google Scholar]
- Koster, K.L.; Webb, M.S.; Bryant, G.; Lynch, D.V. Interactions between soluble sugars and POPC (1-palmitoyl-2-oleoylphosphatidylcholine) during dehydration: vitrification of sugars alters the phase behavior of the phospholipid. Biochim. Biophys. Acta 1994, 1193, 143–150. [Google Scholar] [CrossRef]
- Wolfe, J.; Bryant, G. Freezing, drying, and/or vitrification of membrane- solute-water systems. Cryobiology 1999, 39, 103–129. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The Role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Sun, W.Q.; Leopold, A.C. Cytoplasmic vitrification and survival of anhydrobiotic organisms. Comp. Biochem. Physiol. A 1997, 117, 327–333. [Google Scholar] [CrossRef]
- Williams, R.J.; Leopold, A.C. The glassy state in corn embryos. Plant Physiol. 1989, 89, 977–981. [Google Scholar] [CrossRef]
- Sun, W.Q.; Irving, T.C.; Leopold, A.C. The role of sugar, vitrification and membrane phase transition in seed desiccation tolerance. Physiol. Plant. 1994, 90, 621–628. [Google Scholar] [CrossRef]
- Green, J.L.; Angell, C.A. Phase relations and vitrification in saccharide-water solutions and the trehalose anomaly. J. Phys. Chem. 1989, 93, 2880–2882. [Google Scholar] [CrossRef]
- Crowe, L.M.; Reid, D.S.; Crowe, J.H. Is trehalose special for preserving dry biomaterials? Biophys. J. 1996, 71, 2087–2093. [Google Scholar] [CrossRef]
- Sun, W.Q.; Leopold, A.C.; Crowe, L.M.; Crowe, J.H. Stability of dry liposomes in sugar glasses. Biophys. J. 1996, 70, 1769–1776. [Google Scholar] [CrossRef]
- Crowe, J.H.; Oliver, A.E.; Tablin, F. Is there a single biochemical adaptation to anhydrobiosis? Integr. Comp. Biol. 2002, 42, 497–503. [Google Scholar] [CrossRef]
- Sum, A.K.; Faller, R.; de Pablo, J.J. Molecular simulation study of phospholipid bilayers and insights of the interactions with disaccharides. Biophys. J. 2003, 85, 2830–2844. [Google Scholar] [CrossRef]
- Pereira, C.S.; Lins, R.D.; Chandrasekhar, I.; Freitas, L.C.G.; Hünenberger, P.H. Interaction of the disaccharide trehalose with a phospholipid bilayer: a molecular dynamics study. Biophys. J. 2004, 86, 2273–2285. [Google Scholar] [CrossRef]
- Cottone, G.; Cordone, L.; Ciccotti, G. Molecular dynamics simulation of carboxy-myoglobin embedded in a trehalose-water matrix. Biophys. J. 2001, 80, 931–938. [Google Scholar] [CrossRef]
- Cottone, G.; Ciccotti, G.; Cordone, L. Protein-trehalose-water structures in trehalose coated carboxy-myoglobin. J. Chem. Phys. 2002, 117, 9862–9866. [Google Scholar] [CrossRef]
- Lins, R.D.; Pereira, C.S.; Hünenberger, P.H. Trehalose-protein interaction in aqueous solution. Proteins 2004, 55, 177–186. [Google Scholar] [CrossRef]
- Koynova, R.; Brankov, J.; Tenchov, B. Modulation of lipid phase behavior by kosmotropic and chaotropic solutes. Eur. Biophys. J. 1997, 25, 261–274. [Google Scholar] [CrossRef]
- Koynova, R.D.; Tenchov, B.G.; Quinn, P.J. Sugars favour formation of hexagonal (HII) phase at the expense of lamellar liquid-crystalline phase in hydrated phosphatidylethanolamines. Biochim. Biophys. Acta 1989, 980, 377–380. [Google Scholar] [CrossRef]
- Branca, C.; Magazu, S.; Maisano, G.; Migliardo, P. Anomalous cryoprotective effectiveness of trehalose: Raman scattering evidences. J. Chem. Phys. 1999, 111, 281–287. [Google Scholar] [CrossRef]
- Branca, C.; Maccarrone, S.; Magazu, S.; Maisano, G.; Bennington, S.M.; Taylor, J. Tetrahedral order in homologous disaccharide-water mixtures. J. Chem. Phys. 2005, 122, 174513. [Google Scholar] [CrossRef]
- Sola-Penna, M.; Meyer-Fernandes, J.R. Stabilization against thermal inactivation promoted by sugars on enzyme structure and function: Why is trehalose more effective than other sugars? Arch. Biochem. Biophys. 1998, 360, 10–14. [Google Scholar] [CrossRef]
- Beilinsson, A. Thermostabilität der eiweißlösungen mit rohrzucker und glyzerin. Biochem. Z. 1929, 213, 399–405. [Google Scholar]
- Shikama, K.; Yamazaki, T. Denaturation of catalase by freezing and thawing. Nature 1961, 190, 83–84. [Google Scholar] [CrossRef]
- Tanford, C.; Buckley, C.E., III; De, P.K.; Lively, E.P. Effect of ethylene glycol on the conformation of γ-globulin and β-lactoglobulin. J. Biol. Chem. 1962, 237, 1168–1171. [Google Scholar]
- Gerlsma, S.Y. Reversible denaturation of ribonuclease in aqueous solutions as influenced by polyhydric alcohols and some other additives. J. Biol. Chem. 1968, 243, 957–961. [Google Scholar]
- Hanafusa, N. Denaturation of enzyme protein by freeze-thawing and freeze-drying. In Freezing and Drying of Microorganisms; Nei, T., Ed.; Univ. Tokyo Press: Tokyo, Japan, 1969; pp. 117–129. [Google Scholar]
- Bradbury, S.L.; Jakoby, W.B. Glycerol as an enzyme-stabilizing agent: effects on aldehyde dehydrogenase. Proc. Natl. Acad. Sci. USA 1972, 69, 2373–6. [Google Scholar] [CrossRef]
- Back, J.F.; Oakenfull, D.; Smith, M.B. Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry 1979, 18, 5191–5196. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. Stabilization of protein structure by sugars. Biochemistry 1982, 21, 6536–6544. [Google Scholar] [CrossRef]
- Hellman, K.; Miller, D.S.; Cammack, K.A. The effect of freeze-drying on the quaternary structure of l-asparaginase from Erwinia carotovora. Biochim. Biophys. Acta 1983, 749, 133–142. [Google Scholar] [CrossRef]
- Sun, W.Q.; Davidson, P.; Chan, H.S.O. Protein stability in the amorphous carbohydrate matrix: relevance to anhydrobiosis. Biochim. Biophys. Acta 1998, 1425, 245–254. [Google Scholar]
- Carpenter, J.F.; Crowe, L.M.; Crowe, J.H. Stabilization of phosphofructokinase with sugars during freeze-drying: characterization of enhanced protection in the presence of divalent cations. Biochim. Biophys. Acta 1987, 923, 109–115. [Google Scholar] [CrossRef]
- Colaço, C.; Sen, S.; Thangavelu, M.; Pinder, S.; Roser, B. Extraordinary stability of enzymes dried in trehalose: Simplified molecular biology. Bio/Technology 1992, 10, 1007–1011. [Google Scholar] [CrossRef]
- Leslie, S.B.l; Israeli, E.; Lighthart, B.; Crowe, J. H.; Crowe, L. M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar]
- Xie, G.; Timasheff, S.N. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 1997, 64, 25–43. [Google Scholar] [CrossRef]
- Mazzobre, M.F.; del Pilar Buera, M.; Chirife, J. Protective Role of Trehalose on Thermal Stability of Lactase in Relation to its Glass and Crystal Forming Properties and Effect of Delaying Crystallization. Lebensm. Wiss. Technol. 1997, 30, 324–329. [Google Scholar] [CrossRef]
- Carninci, P.; Nishiyama, Y.; Westover, A.; Itoh, M.; Nagaoka, S.; Sasaki, N.; Okazaki, Y.; Muramatsu, M.; Hayashizaki, Y. Thermostabilization and thermoactivation of thermolabile enzymes by trehalose and its application for the synthesis of full length cDNA. Proc. Natl. Acad. Sci. USA 1998, 95, 520–524. [Google Scholar] [CrossRef]
- Singer, M.A.; Lindquist, S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1998, 1, 639–648. [Google Scholar] [CrossRef]
- Zancan, P.; Sola-Penna, M. Trehalose and glycerol stabilize and renature yeast inorganic pyrophosphatase inactivated by very high temperatures. Arch. Biochem. Biophys. 2005, 444, 52–60. [Google Scholar] [CrossRef]
- Costantino, H.R.; Laleh, F.; Wu, C.; Carrasquillo, K.G.; Griebenow, K.; Zale, S.E.; Tracy, M.A. Protein spray freeze drying. 2. Effect of formulation variables on particle size and stability. J. Pharm. Sci. 2002, 91, 388–395. [Google Scholar] [CrossRef]
- Arakawa, T.; Ejima, D.; Kita, Y.; Tsumoto, K. Small molecule pharmacological chaperones: From thermodynamic stabilization to pharmaceutical drugs. Biochim. Biophys. Acta 2006, 1764, 1677–1687. [Google Scholar]
- Welch, W.J.; Brown, C.R. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1996, 1, 109–15. [Google Scholar] [CrossRef]
- Crowe, J.H. Trehalose as a "chemical chaperone": fact and fantasy. Adv. Exp. Med. Biol. 2007, 594, 143–158. [Google Scholar] [CrossRef]
- Arora, A.; Ha, C.; Park, C.B. Inhibition of insulin amyloid formation by small stress molecules. FEBS Lett. 2004, 564, 121–125. [Google Scholar] [CrossRef]
- Tanaka, M.; Machida, Y.; Niu, S.; Ikeda, T.; Jana, N. R.; Doi, H.; Kurosawa, M.; Nekooki, M.; Nukina, N. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat. Med. 2004, 10, 148–154. [Google Scholar] [CrossRef]
- Davies, J.E.; Sarkar, S.; Rubinsztein, D.C. Trehalose reduces aggregate formation and delays pathology in a transgenic mouse model of oculopharyngeal muscular dystrophy. Hum. Mol. Genet. 2006, 15, 23–31. [Google Scholar]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10 (Suppl.), S10–S17. [Google Scholar] [CrossRef]
- Liu, R.; Barkhordarian, H.; Emadi, S.; Park, C.B.; Sierks, M.R. Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol. Dis. 2005, 20, 74–81. [Google Scholar] [CrossRef]
- Attanasio, F.; Cascio, C.; Fisichella, S.; Nicoletti, V.G.; Pignataro, B.; Savarino, A.; Rizzarelli, E. Trehalose effects on α-crystallin aggregates. Biochem. Biophys. Res. Commun. 2007, 354, 899–905. [Google Scholar] [CrossRef]
- Vilasi, S.; Iannuzzi, C.; Portaccio, M.; Irace, G.; Sirangelo, I. Effect of trehalose on W7FW14F apomyoglobin and insulin fibrillization: New insight into inhibition activity. Biochemistry 2008, 47, 1789–1796. [Google Scholar] [CrossRef]
- Fleming, K.K.; Hubel, A. Cryopreservation of hematopoietic stem cells: emerging science, technology and issues. Transfus. Med. Hemother. 2007, 34, 268–275. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Oliver, A.E.; Tsvetkova, N.; Wolkers, W.; Tablin, F. The trehalose myth revisited: Introduction to a symposium on stabilization of cells in the dry state. Cryobiology 2001, 43, 89–105. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar]
- Tunnacliffe, A.; García de Castro, A.; Manzanera, M. Anhydrobiotic engineering of bacterial and mammalian cells: Is intracellular trehalose sufficient? Cryobiology 2001, 43, 124–132. [Google Scholar] [CrossRef]
- Coutinho, C.; Bernardes, E.; Félix, D.; Panek, A.D. Trehalose as cryoprotectant for preservation of yeast strains. J. Biotechnol. 1988, 7, 23–32. [Google Scholar] [CrossRef]
- Eleutheria, E.C.A.; de Araujo, P.S.; Panek, A.D. Role of the trehalose carrier in dehydration resistance of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1993, 1156, 263–266. [Google Scholar] [CrossRef]
- Beattie, G.M.; Crowe, J.H.; Lopez, A.D.; Cirulli, V.; Ricordi, C.; Hayek, A. Trehalose: a cryoprotectant that enhances recovery and preserves function of human pancreatic islets after long-term storage. Diabetes 1997, 46, 519–523. [Google Scholar] [CrossRef]
- Eroglu, A.; Russo, M. J.; Bieganski, R.; Fowler, A.; Cheley, S.; Bayley, H.; Toner, M. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol. 2000, 18, 163–167. [Google Scholar] [CrossRef]
- Guo, N.; Puhlev, I.; Brown, D.R.; Mansbridge, J.; Levine, F. Trehalose expression confers desiccation tolerance on human cells. Nat. Biotechnol. 2000, 18, 168–171. [Google Scholar] [CrossRef]
- Chen, T.; Acker, J.P.; Eroglu, A.; Cheley, S.; Bayley, H.; Fowler, A.; Toner, M. Beneficial effect of intracellular trehalose on the membrane integrity of dried mammalian cells. Cryobiology 2001, 43, 168–181. [Google Scholar] [CrossRef]
- Acker, J.P.; Fowler, A.; Lauman, B.; Cheley, S.; Toner, M. Survival of desiccated mammalian cells: Beneficial effects of isotonic media. Cell Preserv. Technol. 2002, 1, 129–140. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Walker, N.J.; Tablin, F.; Crowe, J.H. Human platelets loaded with trehalose survive freeze-drying. Cryobiology 2001, 42, 79–87. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Suzuki, S.; Nomura, K.; Enosawa, S. Improvement of hepatocyte viability after cryopreservation by supplementation of long-chain oligosaccharide in the freezing medium in rats and humans. Cell Transpl. 2006, 15, 911–919. [Google Scholar] [CrossRef]
- Katenz, E.; Vondran, F.W.R.; Schwartlander, R.; Pless, G.; Gong, X.; Cheng, X.; Neuhaus, P.; Sauer, I.M. Cryopreservation of primary human hepatocytes: The benefit of trehalose as an additional cryoprotective agent. Liver Transpl. 2007, 13, 38–45. [Google Scholar] [CrossRef]
- Eroglu, A.; Toner, M.; Toth, T.L. Beneficial effect of microinjected trehalose on the cryosurvival of human oocytes. Fertil. Steril. 2002, 77, 152–158. [Google Scholar] [CrossRef]
- Torok, Z.; Satpathy, G.R.; Banerjee, M.; Bali, R.; Little, E.; Novaes, R.; Ly, H.V.; Dwyre, D.M.; Kheirolomoom, A.; Tablin, F.; Crowe, J.H.; Tsvetkova, N.M. Preservation of trehalose-loaded red blood cells by lyophilization. Cell Preserv. Technol. 2005, 3, 96–111. [Google Scholar] [CrossRef]
- Limaye, L.S.; Kale, V.P. Cryopreservation of human hematopoietic cells with membrane stabilizers and bioantioxidants as additives in the conventional freezing medium. J. Hematother. Stem Cell Res. 2001, 10, 709–718. [Google Scholar] [CrossRef]
- Buchanan, S.S.; Gross, S.A.; Acker, J.P.; Toner, M.; Carpenter, J.F.; Pyatt, D.W. Cryopreservation of stem cells using trehalose: Evaluation of the method using a human hematopoietic cell line. Stem Cells Dev. 2004, 13, 295–305. [Google Scholar] [CrossRef]
- Ji, L.; de Pablo, J.J.; Palecek, S.P. Cryopreservation of adherent human embryonic stem cells. Biotechnol. Bioeng. 2004, 88, 299–312. [Google Scholar] [CrossRef]
- Wu, C.F.; Tsung, H.C.; Zhang, W.J.; Wang, Y.; Lu, J.H.; Tang, Z.Y.; Kuang, Y.P.; Jin, W.; Cui, L.; Liu, W.; Cao, Y.L. Improved cryopreservation of human embryonic stem cells with trehalose. Reprod. Biomed. Online 2005, 11, 733–739. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Andreyev, A.; Murphy, A.N.; Perkins, G.A.; Ellisman, M.H.; Newmeyer, D.D. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell. Death Differ. 2006, 14, 616–624. [Google Scholar]
- Pu, L.L.Q.; Cui, X.; Fink, B.F.; Cibull, M.L.; Gao, D. Cryopreservation of adipose tissues: The role of trehalose. Aesthetic Surg. J. 2005, 25, 126–131. [Google Scholar] [CrossRef]
- Matsuo, T. Trehalose protects corneal epithelial cells from death by drying. Br. J. Ophthalmol. 2001, 85, 610–612. [Google Scholar] [CrossRef]
- Matsuo, T.; Tsuchida, Y.; Morimoto, N. Trehalose eye drops in the treatment of dry eye syndrome. Ophthalmology 2002, 109, 2024–2029. [Google Scholar] [CrossRef]
- Puhlev, I.; Guo, N.; Brown, D.R.; Levine, F. Desiccation tolerance in human cells. Cryobiology 2001, 42, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Hirata, T.; Yokomise, H.; Fukuse, T.; Muro, K.; Inui, K.; Yagi, K.; Hitomi, S.; Wada, H. Effects of trehalose in preservation of canine lung for transplants. Thorac. Cardiovasc. Surg. 1993, 41, 59–63. [Google Scholar] [CrossRef]
- Wada, H.; Liu, C.J.; Hirata, T.; Bando, T.; Kosaka, S. Effective 30-hour preservation of canine lungs with modified ET-Kyoto solution. Ann. Thorac. Surg. 1996, 61, 1099–1105. [Google Scholar] [CrossRef]
- Chen, F.; Nakamura, T.; Wada, H. Development of new organ preservation solutions in Kyoto University. Yonsei Med. J. 2004, 45, 1107–1114. [Google Scholar]
- Zhao, X.; Koshiba, T.; Nakamura, T.; Tsuruyama, T.; Li, Y.; Bando, T.; Wada, H.; Tanaka, K. ET-Kyoto solution plus dibutyryl cyclic adenosine monophosphate is superior to University of Wisconsin solution in rat liver preservation. Cell Transpl. 2008, 17, 99–109. [Google Scholar] [CrossRef]
- Gribbon, E.M.; Sen, S.D.; Roser, B.J.; Kampinga, J. Stabilisation of vaccines using trehalose (Q-T4) technology. Dev. Biol. Stand. 1996, 87, 193–199. [Google Scholar]
- Aguado, M.T.; Jodar, L.; Lloyd, J.; Lambert, P.H. Injectable solid vaccines: A role in future immunization? WHO Drug info. 1998, 12, 68–69. [Google Scholar]
- Quintilio, W.; Sato, R.A.; Sant'Anna, O.A.; Esteves, M.I.; Sesso, A.; de Araujo, P.S.; Bueno da Costa, M.H. Large unilamellar vesicles as trehalose-stabilised vehicles for vaccines: storage time and in vivo studies. J. Control. Release 2000, 67, 409–413. [Google Scholar] [CrossRef]
- Bullifent, H.L.; Dhaliwal, K.; Howells, A.M.; Goan, K.; Griffin, K.; Lindsay, C.D.; Tunnacliffe, A.; Titball, R.W. Stabilisation of Salmonella vaccine vectors by the induction of trehalose biosynthesis. Vaccine 2000, 19, 1239–1245. [Google Scholar] [CrossRef]
- Esteves, M.I.; Quintilio, W.; Sato, R.A.; Raw, I.; Soares de Araujo, P.; Bueno da Costa, M.H. Stabilisation of immunoconjugates by trehalose. Biotechnol. Lett. 2000, 22, 417–420. [Google Scholar] [CrossRef]
- Cleland, J.L.; Lam, X.; Kendrick, B.; Yang, J.; Yang, T.-H.; Overcashier, D.; Brooks, D.; Hsu, C.; Carpenter, J.F. A specific molar ratio of stabilizer to protein is required for storage stability of a lyophilized monoclonal antibody. J. Pharm. Sci. 2001, 90, 310–321. [Google Scholar] [CrossRef]
- Blakeley, D.; Roser, B.; Tolliday, B.; Colaço, C. Dry instant blood typing plate for bedside use. Lancet 1990, 336, 854–855. [Google Scholar] [CrossRef]
- Arya, S.C. Stabilization of vaccines: to be or not to be. Vaccine 2000, 19, 595–597. [Google Scholar] [CrossRef]
- Schiraldi, C.; Di Lernia, I.; De Rosa, M. Trehalose production: exploiting novel approaches. Trends Biotechnol. 2002, 20, 420–425. [Google Scholar] [CrossRef]
- Kubota, M.; Sawatani, I.; Oku, K.; Takeuchi, K.; Murai, S. The development of α,α-trehalose production and its applications. J. Appl. Glycosci. 2004, 51, 63–70. [Google Scholar] [CrossRef]
- Nishizaki, Y.; Yoshizane, C.; Toshimori, Y.; Arai, N.; Akamatsu, S.; Hanaya, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Disaccharide-trehalose inhibits bone resorption in ovariectomized mice. Nutr. Res. 2000, 20, 653–664. [Google Scholar] [CrossRef]
- Yoshizane, C.; Arai, N.; Arai, C.; Yamamoto, M.; Nishizaki, Y.; Hanaya, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Trehalose suppresses osteoclast differentiation in ovariectomized mice: Correlation with decreased in vitro interleukin-6 production by bone marrow cells. Nutr. Res. 2000, 20, 1485–1491. [Google Scholar]
- Arai, C.; Kohguchi, M.; Akamatsu, S.; Arai, N.; Yoshizane, C.; Hasegawa, N.; Hanaya, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Trehalose suppresses lipopolysaccharide-induced osteoclastogenesis bone marrow in mice. Nutr. Res. 2001, 21, 993–999. [Google Scholar] [CrossRef]
- Otsubo, M.; Iwaya-Inoue, M. Trehalose delays senescence in cut gladiolus spikes. HortSci. 2000, 35, 1107–1110. [Google Scholar]
- Birch, G.G. Trehalose. Adv. Carbohydr. Chem. 1963, 18, 201–225. [Google Scholar]
- Fischer, E.; Delbrück, K. Synthese neuer Disaccharide vom Typus der Trehalose. Ber. 1909, 42, 2776–2785. [Google Scholar] [CrossRef]
- Helferich, B.; Weis, K. Zur Synthese von Glucosiden und von nicht-reduzierenden Disacchariden. Chem. Ber. 1956, 89, 314–321. [Google Scholar] [CrossRef]
- Vogel, H.; Debowska-Kurnicka, H. Sur quelques nouveaux sucres du type du tréhalose. Helv. Chim. Acta 1928, 11, 910–915. [Google Scholar] [CrossRef]
- Sharp, V.E.; Stacey, M. 63. Synthesis of trehalose-type disaccharides. J. Chem. Soc. 1951, 285–288. [Google Scholar] [CrossRef]
- Frahm, H. Über das Polykondensationsgleichgewicht von d-Glucose in Gegenwart von Chlorwasserstoff als Katalysator. Justus Liebigs Ann. Chem. 1944, 555, 187–213. [Google Scholar] [CrossRef]
- Lemieux, R.U.; Bauer, H.F. A chemical synthesis of d-trehalose. Can. J. Chem. 1954, 32, 340–344. [Google Scholar] [CrossRef]
- Montgomery, E.M.; Weakly, F.B. Carbohydrate composition of hydrol. J. Assoc. Offic. Agr. Chemists 1953, 36, 1096–1108. [Google Scholar]
- Haq, S.; Whelan, W.J. The chemical synthesis of polysaccharides. Part II. The chemical synthesis of nigerose. J. Chem. Soc. 1958, 1342–1346. [Google Scholar]
- Haines, A.H. Synthesis of l-trehalose and observations on isomer and by-product formation. Carbohydr. Res. 2003, 338, 813–818. [Google Scholar] [CrossRef]
- Haines, A.H. Non-equivalence of d- and l-trehalose in stabilising alkaline phosphatase against freeze-drying and thermal stress. Is chiral recognition involved? Org. Biomol. Chem. 2006, 4, 702–706. [Google Scholar] [CrossRef]
- Lin, F.L.; van Halbeek, H.; Bertozzi, C.R. Synthesis of mono- and dideoxygenated α,α-trehalose analogs. Carbohydr. Res. 2007, 342, 2014–2030. [Google Scholar]
- Leloir, L.F.; Cabib, E. The enzymic synthesis of trehalose phosphate. J. Am. Chem. Soc. 1953, 75, 5445–5446. [Google Scholar] [CrossRef]
- Cabib, E.; Leloir, L.F. The biosynthesis of trehalose phosphate. J. Biol. Chem. 1958, 231, 259–275. [Google Scholar]
- Elbein, A.D. Carbohydrate metabolism in Streptomyces hygroscopicus. I. Enzymatic synthesis of trehalose phosphate from guanosine diphosphate d-glucose-14C. J. Biol. Chem. 1967, 242, 403–406. [Google Scholar]
- Lapp, D.; Patterson, B.W.; Elbein, A.D. Properties of a trehalose phosphate synthetase from Mycobacterium smegmatis. Activation of the enzyme by polynucleotides and other polyanions. J. Biol. Chem. 1971, 246, 4567–4579. [Google Scholar]
- Gibson, R.P.; Turkenburg, J.P.; Charnock, S.J.; Lloyd, R.; Davies, G.J. Insights into trehalose synthesis provided by the structure of the retaining glucosyltransferase OtsA. Chem. Biol. 2002, 9, 1337–1346. [Google Scholar] [CrossRef]
- Friedman, S. Occurrence of trehalose-6-phosphatase in Phormia regina Meig. Arch. Biochem. Biophys. 1960, 88, 339–343. [Google Scholar]
- Matula, M.; Mitchell, M.; Elbein, A.D. Partial purification and properties of a highly specific trehalose phosphate phosphatase from Mycobacterium smegmatis. J. Bacteriol. 1971, 107, 217–222. [Google Scholar]
- Rao, K.N.; Kumaran, D.; Seetharaman, J.; Bonanno, J.B.; Burley, S.K.; Swaminathan, S. Crystal structure of trehalose-6-phosphate phosphatase-related protein: Biochemical and biological implications. Protein Sci. 2006, 15, 1735–1744. [Google Scholar] [CrossRef]
- Kaasen, I.; Falkenberg, P.; Styrvold, O.B.; Strom, A.R. Molecular cloning and physical mapping of the otsBA genes, which encode the osmoregulatory trehalose pathway of Escherichia coli: evidence that transcription is activated by katF (AppR). J. Bacteriol. 1992, 174, 889–898. [Google Scholar]
- Strøm, A.R.; Kaasen, I. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol. Microbiol. 1993, 8, 205–210. [Google Scholar] [CrossRef]
- Bell, W.; Klaassen, P.; Ohnacker, M.; Boller, T.; Herweijer, M.; Schoppink, P.; Vanderzee, P.; Wiemken, A. Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. Eur. J. Biochem. 1992, 209, 951–959. [Google Scholar] [CrossRef]
- Virgilio, C.; Bürckert, N.; Bell, W.; Jenö, P.; Boller, T.; Wiemken, A. Disruption of TPS2, the gene encoding the 100-kDa subunit of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae, causes accumulation of trehalose-6-phosphate and loss of trehalose-6-phosphate phosphatase activity. Eur. J. Biochem. 1993, 212, 315–323. [Google Scholar] [CrossRef]
- Bell, W.; Sun, W.; Hohmann, S.; Wera, S.; Reinders, A.; De Virgilio, C.; Wiemken, A.; Thevelein, J.M. Composition and functional analysis of the Saccharomyces cerevisiae trehalose synthase complex. J. Biol. Chem. 1998, 273, 33311–33319. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Terry, N.; Sears, T.; Kim, H.; Zayed, A.; Hwang, S.; Van Dun, K.; Voogd, E.; Verwoerd, T. C.; Krutwagen, R. Trehalose-producing transgenic tobacco plants show improved growth performance under drought stress. J. Plant Physiol. 1998, 152, 525–532. [Google Scholar] [CrossRef]
- Romero, C.; Bellés, J.M.; Vayá, J.L.; Serrano, R.; Culiáñez-Macià, F.A. Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta 1997, 201, 293–297. [Google Scholar] [CrossRef]
- Pellny, T.K.; Ghannoum, O.; Conroy, J.P.; Schluepmann, H.; Smeekens, S.; Andralojc, J.; Krause, K.P.; Goddijn, O.; Paul, M.J. Genetic modification of photosynthesis with E. coli genes for trehalose synthesis. Plant Biotechnol. J. 2004, 2, 71–82. [Google Scholar] [CrossRef]
- Schluepmann, H.; Pellny, T.; van Dijken, A.; Smeekens, S.; Paul, M. Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6849–6854. [Google Scholar] [CrossRef]
- Karim, S.; Aronsson, H.; Ericson, H.; Pirhonen, M.; Leyman, B.; Welin, B.; Mäntylä, E.; Palva, E.; Van Dijck, P.; Holmström, K.-O. Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol. Biol. 2007, 64, 371–386. [Google Scholar] [CrossRef]
- Chary, S.N.; Hicks, G.R.; Choi, Y.G.; Carter, D.; Raikhel, N.V. Trehalose-6-phosphate synthase/phosphatase regulates cell shape and plant architecture in Arabidopsis. Plant Physiol. 2008, 146, 97–107. [Google Scholar]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y. Trehalose metabolism and signaling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef]
- Nishimoto, T.; Nakano, M.; Ikegami, S.; Chaen, H.; Fukuda, S.; Sugimoto, T.; Kurimoto, M.; Tsujisaka, Y. Existence of a novel enzyme converting maltose into trehalose. Biosci. Biotechnol. Biochem. 1995, 59, 2189–2190. [Google Scholar] [CrossRef]
- Nishimoto, T.; Nakada, T.; Chaen, H.; Fukuda, S.; Sugimoto, T.; Kurimoto, M.; Tsujisaka, Y. Purification and characterization of a thermostable trehalose synthase from Thermus aquaticus. Biosci. Biotechnol. Biochem. 1996, 60, 835–839. [Google Scholar] [CrossRef]
- Maruta, K.; Nakada, T.; Kubota, M.; Chaen, H.; Sugimoto, T.; Kurimoto, M.; Tsujisaka, Y. Formation of trehalose from maltooligosaccharides by a novel enzymatic system. Biosci. Biotechnol. Biochem. 1995, 59, 1829–1834. [Google Scholar] [CrossRef]
- Nakada, T.; Maruta, K.; Tsusaki, K.; Kubota, M.; Chaen, H.; Sugimoto, T.; Kurimoto, M.; Tsujisaka, Y. Purification and properties of a novel enzyme, maltooligosyl trehalose synthase, from Arthrobacter sp. Q36. Biosci. Biotechnol. Biochem. 1995, 59, 2210–2214. [Google Scholar] [CrossRef]
- Nakada, T.; Maruta, K.; Mitsuzumi, H.; Kubota, M.; Chaen, H.; Sugimoto, T.; Kurimoto, M.; Tsujisaka, Y. Purification and characterization of a novel enzyme, maltooligosyl trehalose trehalohydrolase, from Arthrobacter sp. Q36. Biosci. Biotechnol. Biochem. 1995, 59, 2215–2218. [Google Scholar] [CrossRef]
- Maruta, K.; Hattori, K.; Nakada, T.; Kubota, M.; Sugimoto, T.; Kurimoto, M. Cloning and sequencing of trehalose biosynthesis genes from Arthrobacter sp. Q36. Biochim. Biophys. Acta 1996, 1289, 10–13. [Google Scholar] [CrossRef]
- Maruta, K.; Kubota, M.; Fukuda, S.; Kurimoto, M. Cloning and nucleotide sequence of a gene encoding a glycogen debranching enzyme in the trehalose operon from Arthrobacter sp. Q36. Biochim. Biophys. Acta 2000, 1476, 377–381. [Google Scholar] [CrossRef]
- Nakada, T.; Ikegami, S.; Chaen, H.; Kubota, M.; Fukuda, S.; Sugimoto, T.; Kurimoto, M.; Tsujisaka, Y. Purification and characterization of thermostable maltooligosyl trehalose synthase from the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biosci. Biotechnol. Biochem. 1996, 60, 263–266. [Google Scholar] [CrossRef]
- Kato, M.; Miura, Y.; Kettoku, M.; Shindo, K.; Iwamatsu, A.; Kobayashi, K. Purification and characterization of new trehalose-producing enzymes isolated from the hyperthermophilic archae, Sulfolobus solfataricus KM1. Biosci. Biotechnol. Biochem. 1996, 60, 546–550. [Google Scholar] [CrossRef]
- Kidd, G.; Devorak, J. Trehalose is a sweet target for agbiotech. Bio/Technology 1994, 12, 1328–1329. [Google Scholar] [CrossRef]
- Haynie, S.; Whitesides, G. Enzyme-catalyzed organic synthesis of sucrose and trehalose with in situ regeneration of UDP-Glucose. Appl. Biochem. Biotechnol. 1990, 23, 155–170. [Google Scholar] [CrossRef]
- Goddijn, O.J.; Verwoerd, T. C.; Voogd, E.; Krutwagen, R. W.; de Graaf, P. T.; van Dun, K.; Poels, J.; Ponstein, A. S.; Damm, B.; Pen, J. Inhibition of trehalase activity enhances trehalose accumulation in transgenic plants. Plant Physiol. 1997, 113, 181–90. [Google Scholar]
- Londesborough, J.; Tunnela, O.; Holmstrom, K.O.; Mantyla, E.; Welin, B.; Mandal, A.; Palva, T.E. Transgenic plants producing trehalose. US Pat. 6,130,368, 2000. [Google Scholar]
- Lebel, E.G.; Heifetz, P.B.; Goff, S.A. Expression of trehalose 6-phosphate synthase in plant plastids. US Pat. 6,686,516, 2004. [Google Scholar]
- Yamamoto, T.; Maruta, K.; Watanabe, H.; Yamashita, H.; Kubota, M.; Fukuda, S.; Kurimoto, M. Trehalose-producing operon treYZ from Arthrobacter ramosus S34. Biosci. Biotechnol. Biochem. 2001, 65, 1419–1423. [Google Scholar] [CrossRef]
- Sachinvala, N.D.; Ju, R.F.; Litt, M.H.; Niemczura, W.P. Preparation of poly(methyl methacrylate) and copolymers having enhanced thermal stabilities using sucrose-based comonomers and additives. J. Polym. Sci. Part A: Polym. Chem. 1995, 33, 15–29. [Google Scholar] [CrossRef]
- Sachinvala, N.D.; Ju, R.F.; Litt, M.H. Thermostable polymers from 1', 2', 3, 3', 4, 4', 6, 6'-octa-O-allylsucrose. US Pat. 5,470,931, 1995. [Google Scholar]
- Sachinvala, N.D.; Ju, R.F.; Litt, M.H. Polymers having enhanced thermal stabilities and methods for their preparation using 1’,2,3,3’,4,4’,6,6’-octa-O-allylsucrose. US Pat. 5,621,056, 1997. [Google Scholar]
- Sachinvala, N.D.; Ju, R.F.; Litt, M.H. Polymers having enhanced thermal stabilities and methods for their preparation using stabilization agents 1’,6,6’-trimethacryloyl-2,3,3’,4,4’-penta-O-methylsucrose, 1’,2,3,3’,4,4’,6,6’-octa-O-allylsucrose, and 1’,2,3,3’,4,4’,6,6’-Octa-O-crotyl-sucrose. US Pat. 5,789,469, 1998. [Google Scholar]
- Dordick, J.S.; Rethwisch, D.G.; Patil, D.R.; Martin, B.D.; Linhardt, R.J. Sugar-based polymers. US Pat. 5,854,030, 1998. [Google Scholar]
- Elisseeff, J.; Tuan, R.S.; Li, Q.; Wang, D.; Holton, L.H.; Silverman, R.P. Cross-linked polymer matrices, and methods of making and using same. EP Pat. 1,558,216, 2005. [Google Scholar]
- Abe, Y.; Anzai, T. Crosslinkable polysaccharide derivative, process for producing the same, crosslinkable polysaccharide composition, and medical treatment material. EP Pat. 1,595,892, 2005. [Google Scholar]
- Teramoto, N.; Shibata, M. Trehalose-based thermosetting resins. I. Synthesis and thermal properties of trehalose vinylbenzyl ether. J. Appl. Polym. Sci. 2004, 91, 46–51. [Google Scholar] [CrossRef]
- Teramoto, N.; Shibata, M. Synthesis and photocuring of cinnamoyl trehalose esters. Polym. Adv. Technol. 2007, 18, 971–977. [Google Scholar] [CrossRef]
- Minsk, L.M.; Smith, J.G.; van Deusen, W.P.; Wright, J.F. Photosensitive polymers. I. Cinnamate esters of poly(vinyl alcohol) and cellulose. J. Appl. Polym. Sci. 1959, 2, 302–307. [Google Scholar] [CrossRef]
- Watanabe, S.; Kato, M.; Kosakai, S. Preparation and properties of photocrosslinkable poly(2-vinyloxyethyl cinnamate). J. Polym. Sci.: Polym. Chem. 1984, 22, 2801–2808. [Google Scholar] [CrossRef]
- Balaji, R.; Grandea, D.; Nanjundan, S. Photoresponsive polymers having pendant chlorocinnamoyl moieties: synthesis, reactivity ratios and photochemical properties. Polymer 2004, 45, 1089–1099. [Google Scholar] [CrossRef]
- Ichimura, K.; Akita, Y.; Akiyama, H.; Kudo, K.; Hayashi, Y. Photoreactivity of polymers with regioisomeric cinnamate side chains and their ability to regulate liquid crystal alignment. Macromolecules 1997, 30, 903–911. [Google Scholar] [CrossRef]
- Shibaev, V.; Borovsky, A.; Boiko, N. Photoactive liquid crystalline polymer systems with light-controllable structure and optical properties. Prog. Polym. Sci. 2003, 28, 729–836. [Google Scholar]
- Sachinvala, N.D.; Litt, M.H.; Niemczura, W.P.; Williams, J.D. Chemorational design synthesis and characterization of monomers from sucrose. Polym. Prep. 1989, 30, 523–524. [Google Scholar]
- Sachinvala, N.D.; Niemczura, W.P.; Litt, M.H. Monomers from sucrose. Carbohydr. Res. 1991, 218, 237–245. [Google Scholar] [CrossRef]
- Sachinvala, N.D. 6,6'-diamino-6,6'-dideoxy-1',2,3,3',4,4'-hexa-O-methylsucrose. US Pat. 5,120,836, 1992. [Google Scholar]
- Lichtenthaler, F.W.; Peters, S. Carbohydrates as green raw materials for the chemical industry. C. R. Chimie 2004, 7, 65–90. [Google Scholar] [CrossRef]
- Kurita, K.; Hirakawa, N.; Morinaga, H.; Iwakura, Y. Synthetic polymers containing sugar residues, 6. Novel polyurethanes by direct addition polymerization of α,α-trehalose with diisocyanates. Makromol. Chem. 1979, 180, 2769–2773. [Google Scholar] [CrossRef]
- Kurita, K.; Masuda, N.; Aibe, S.; Murakami, K.; Ishii, S.; Nishimura, S. Synthetic carbohydrate polymers containing trehalose residues in the main chain: Preparation and characteristic properties. Macromolecules 1994, 27, 7544–7549. [Google Scholar] [CrossRef]
- Patil, D.R.; Rethwisch, D.G.; Dordick, J.S. Enzymatic synthesis of a sucrose-containing linear polyester in nearly anhydrous organic media. Biotechnol. Bioeng. 1991, 37, 639–646. [Google Scholar] [CrossRef]
- Patil, D.R.; Dordick, J.S.; Rethwisch, D.G. Chemoenzymatic synthesis of novel sucrose-containing polymers. Macromolecules 1991, 24, 3462–3463. [Google Scholar] [CrossRef]
- Therisod, M.; Klibanov, A.M. Facile enzymatic preparation of monoacylated sugars in pyridine. J. Am. Chem. Soc. 1986, 108, 5638–5640. [Google Scholar] [CrossRef]
- Riva, S.; Chopineau, J.; Kieboom, A.P.G.; Klibanov, A.M. Protease-catalyzed regioselective esterification of sugars and related compounds in anhydrous dimethylformamide. J. Am. Chem. Soc. 1988, 110, 584–589. [Google Scholar] [CrossRef]
- Park, O.-J.; Kim, D.-Y.; Dordick, J.S. Enzyme-catalyzed synthesis of sugar-containing monomers and linear polymers. Biotechnol. Bioeng. 2000, 70, 208–216. [Google Scholar] [CrossRef]
- Kitagawa, M.; Chalermisrachai, P.; Fan, H.; Tokiwa, Y. Chemoenzymatic synthesis of biodegradable polymers containing glucobiose branches. Makromol. Chem. Macromol. Symp. 1999, 144, 247–256. [Google Scholar] [CrossRef]
- Miura, Y.; Wada, N.; Nishida, Y.; Mori, H.; Kobayashi, K. Chemoenzymatic synthesis of glycoconjugate polymers starting from nonreducing disaccharides. J. Polym. Sci. Part A: Polym. Chem. 2004, 42, 4598–4606. [Google Scholar] [CrossRef]
- Neri, P.; Nagano, S.I.; Yokoyama, S.; Dohi, H.; Kobayashi, K.; Miura, T.; Inazu, T.; Sugiyama, T.; Nishida, Y.; Mori, H. Neutralizing activity of polyvalent Gb3, Gb2 and galacto-trehalose models against Shiga toxins. Microbial. Immunol. 2007, 51, 581–592. [Google Scholar] [CrossRef]
- Bower, J.R. Foodborne diseases: Shiga toxin producing E. coli (STEC). Pediatr. Infect. Dis. J. 1999, 18, 909–910. [Google Scholar] [CrossRef]
- Beutin, L. Emerging enterohaemorrhagic Escherichia coli, causes and effects of the rise of a human pathogen. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 299–305. [Google Scholar] [CrossRef]
- Gelas, J.; Calinaud, P. Synthesis of Isopropylidene, Benzylidene, and Related Acetals. In Preparative Carbohydrate Chemistry; Hannesian, S., Ed.; Marcel Dekker: New York, 1997; pp. 1–35. [Google Scholar]
- Hough, L.; Munroe, P.A.; Richardson, A.C. Chemical modification of trehalose. Part V. The synthesis of mono-and di-epoxides. J. Chem. Soc. 1971, 1090–1094. [Google Scholar]
- Baer, H.H.; Radatus, B. Preparation of some partially protected, α,α-trehalose-type disaccharides having the -altro configuration. Carbohydr. Res. 1984, 128, 165–174. [Google Scholar] [CrossRef]
- Teramoto, N.; Shibasaki, Y.; Watanabe, D.; Yosomiya, R.; Shibata, M. Synthesis and characterization of the polymer containing trehalose moiety. Polym. Prepr. Jpn. 2002, 51, 1071. [Google Scholar]
- Teramoto, N.; Arai, Y.; Shibasaki, Y.; Shibata, M. A facile synthesis of a novel polyacetal containing trehalose residue in the main chain. Carbohydr. Polym. 2004, 56, 1–6. [Google Scholar] [CrossRef]
- Maslinska-Solich, J. Condensation and polycondensation of terephthaldehyde with methyl d-hexopyranosides. Macromol. Biosci. 2001, 1, 312–321. [Google Scholar] [CrossRef]
- Arai, Y.; Teramoto, N.; Shibata, M. Sythesis and Properties of Novel Liner Polyacetals Containing Trehalose Units. Proceedings of 12th Polymer Material Forum 2003, 12, 42. [Google Scholar]
- Teramoto, N.; Unosawa, M.; Matsushima, S.; Shibata, M. Synthesis and properties of thermoplastic alternating copolymers containing trehalose and siloxane units by hydrosilylation reaction. Polym. J. 2007, 39, 975–981. [Google Scholar] [CrossRef]
- Engle, L.P.; Wagener, K.B. A review of thermally controlled covalent bond formation in polymer chemistry. J. Macromol. Sci.-Rev. Macromol. Chem. Phys. 1993, C33, 239–257. [Google Scholar] [CrossRef]
- Teramoto, N.; Arai, Y.; Shibata, M. Thermo-reversible Diels-Alder polymerization of difurfurylidene trehalose and bismaleimides. Carbohydr. Polym. 2006, 64, 78–84. [Google Scholar] [CrossRef]
- Niwa, M.; Teramoto, N.; Shibata, M. Diels-Alder polymerization of difurfurylidene trehalose and bismaleimide containing siloxane units. Polym. Prepr. Jpn. 2007, 56, 2296. [Google Scholar]
- Srinivasachari, S.; Liu, Y.; Zhang, G.; Prevette, L.; Reineke, T.M. Trehalose click polymers inhibit nanoparticle aggregation and promote pDNA delivery in serum. J. Am. Chem. Soc. 2006, 128, 8176–8184. [Google Scholar] [CrossRef]
- Srinivasachari, S.; Liu, Y.; Prevette, L.E.; Reineke, T.M. Effects of trehalose click polymer length on pDNA complex stability and delivery efficacy. Biomaterials 2007, 28, 2885–2898. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper (I)-catalyzed regioselective "Ligation" of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Teramoto, N.; Abe, Y.; Enomoto, A.; Watanabe, D.; Shibata, M. Novel synthetic route of a trehalose-based linear polymer by ring opening of two epoxy groups with aliphatic diamine. Carbohydr. Polym. 2005, 59, 217–224. [Google Scholar] [CrossRef]
- Sample Availability: Contact the corresponding author via e-mail.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Teramoto, N.; Sachinvala, N.D.; Shibata, M. Trehalose and Trehalose-based Polymers for Environmentally Benign, Biocompatible and Bioactive Materials. Molecules 2008, 13, 1773-1816. https://doi.org/10.3390/molecules13081773
Teramoto N, Sachinvala ND, Shibata M. Trehalose and Trehalose-based Polymers for Environmentally Benign, Biocompatible and Bioactive Materials. Molecules. 2008; 13(8):1773-1816. https://doi.org/10.3390/molecules13081773
Chicago/Turabian StyleTeramoto, Naozumi, Navzer D. Sachinvala, and Mitsuhiro Shibata. 2008. "Trehalose and Trehalose-based Polymers for Environmentally Benign, Biocompatible and Bioactive Materials" Molecules 13, no. 8: 1773-1816. https://doi.org/10.3390/molecules13081773
APA StyleTeramoto, N., Sachinvala, N. D., & Shibata, M. (2008). Trehalose and Trehalose-based Polymers for Environmentally Benign, Biocompatible and Bioactive Materials. Molecules, 13(8), 1773-1816. https://doi.org/10.3390/molecules13081773





