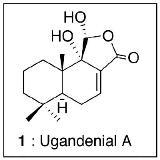
Ugandenial A, a New Drimane-type Sesquiterpenoid from Warburgia ugandensis
Abstract
:Introduction
Results and Discussion
Experimental
General
Plant material
Cytotoxicity activity assay
Extraction and isolation
Ugandenial A (1)
Conclusions
Acknowledgements
References and Notes
- Dharani, N. Field Guide to Common Trees & Shrubs of East Africa; Struik Publishers: Cape Town, South Africa, 2002. [Google Scholar]
- Ssegawa, P.; Kasenene, J.M. Medicinal plant diversity and uses in the Sango bay area, Southern Uganda. J. Ethnopharmacol. 2007, 113, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Heine, B.; König, C. Cologne Development Studies. In Plant concepts and plant use. An ethnobotanical survey of the semi-arid and arid lands of East Africa. Part 2: Plants of the So (Uganda); Verlag breitenbach Publishers: Fort Lauderdale/Saarbrücken, USA/Germany, 1988. [Google Scholar]
- Brooks, C.J.W.; Draffan, G.H. Sesquiterpenoids of Warburgia species-II ugandensolide and ugandansidial (cinnamodial). Tetrahedron 1969, 25, 2887–2898. [Google Scholar] [CrossRef]
- Kioy, D.; Gray, A.I.; Waterman, P.G. A comparative study of the stem-bark drimane sesquiterpenes and leaf volatile oils of Warburgia ugandensis and W. stuhlmannii. Phytochemistry 1990, 29, 3535–3538. [Google Scholar] [CrossRef]
- Wube, A.A.; Franz, B.; Gibbons, S.; Asres, K. Sesquiterpenes from Warburgia ugandensis and their antimycobacterial activity. Phytochemistry 2005, 66, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Lee, Y.W.; Pettei, M.J.; Pilkiewicz, F.; Nakanishi, K. Potent army worm antifeedants from the East African Warburgia plants. Chem. Commun. 1976, 24, 1013–1014. [Google Scholar] [CrossRef]
- Manguro., L.O.A.; Ugi, I.; Lemmen, P.; Hermann, R. Flavonol glycosides of Warburgia ugandensis leaves. Phytochemistry 2003, 64, 891–896. [Google Scholar] [CrossRef]
- Madikane, V.E.; Bhakta, S.; Russell, A.J.; Campbell, W.E.; Claridge, T.D.W.; Elisha, G.; Davies, S.G.D.; Smith, P.; Sim, E. Inhibition of mycobacterial arylamine N-acetyltransferase contributes to anti-mycobacterial activity of Warburgia salutaris. Bioorgan. Med. Chem. 2007, 15, 3579–3586. [Google Scholar] [CrossRef] [PubMed]
- Kioy, D.; Gray, A.I.; Waterman, P.G. Further drimane sesquiterpenes from the stem bark of Canella winterana. J. Nat. Prod. 1989, 52, 174–177. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Sato, T.; Miura, I.; Asakawa, Y. Drimane-type sesqui- and norsesquirterpenoids from Polygonum hydropiper. Phytochemistry 1985, 24, 1521–1524. [Google Scholar] [CrossRef]
- Sakio, Y.; Hirano, Y.J.; Hayashi, M.; Komiyama, K.; Ishibashi, M. Dendocarbins A.-N, new drimane sequiterpenes from the nudibranch Dendrodoris carbunculosa. J. Nat. Prod. 2001, 64, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Allouche, N.; Apel, C.; Martin, M.T.; Dumontet, V.; Guéritte, F.; Litaudon, M. Cytotoxic sesquiterpenoids from Winteraceae of Caledonian rainforest. Phytochemistry 2009, 70, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Tempete, C.; Werner, G.H.; Favre, F.; Rojas, A.; Langlois, N. In vitro cytostatic activity of 9-demethoxyporothramycin B. Eur. J. Med. Chem. 1995, 30, 647–650. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Position | δC | 1 a |
|---|---|---|
| δH (J in Hz) | ||
| 1 | 30.3 | 1.62 (dd, 11.8, 11.8) 1.20 (m) |
| 2 | 17.5 | 1.46 (ddd, 11.8, 11.8, 11.8) 1.37 (m) |
| 3 | 41.1 | 1.35 (m) 1.14 (dd, 11.8, 11.8) |
| 4 | 33.0 | - |
| 5 | 41.1 | 1.79 (dd, 10.7, 5.0) |
| 6-β 6-α | 25.2 | 2.07 (dd, 19.3, 10.7 Hz) 2.32 (dd, 19.3, 5.0 Hz) |
| 7 | 143.3 | 6.98 (brs) |
| 8 | 129.2 | - |
| 9 | 75.7 | - |
| 10 | 39.2 | - |
| 11 | 98.5 | 5.66 (d, 10 Hz) |
| 12 | 168.7 | - |
| 13 | 16.1 | 0.80 (s) |
| 14 | 33.0 | 0.85 (s) |
| 15 | 21.3 | 0.87 (s) |
| 9-OH | 5.00 | |
| 11-OH | 5.90 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, M.; Litaudon, M.; Krief, S.; Martin, M.-T.; Kasenene, J.; Kiremire, B.; Dumontet, V.; Guéritte, F. Ugandenial A, a New Drimane-type Sesquiterpenoid from Warburgia ugandensis. Molecules 2009, 14, 3844-3850. https://doi.org/10.3390/molecules14103844
Xu M, Litaudon M, Krief S, Martin M-T, Kasenene J, Kiremire B, Dumontet V, Guéritte F. Ugandenial A, a New Drimane-type Sesquiterpenoid from Warburgia ugandensis. Molecules. 2009; 14(10):3844-3850. https://doi.org/10.3390/molecules14103844
Chicago/Turabian StyleXu, Min, Marc Litaudon, Sabrina Krief, Marie-Thérèse Martin, John Kasenene, Bernard Kiremire, Vincent Dumontet, and Françoise Guéritte. 2009. "Ugandenial A, a New Drimane-type Sesquiterpenoid from Warburgia ugandensis" Molecules 14, no. 10: 3844-3850. https://doi.org/10.3390/molecules14103844