Study of the Chemical Composition of the Resinous Exudate Isolated from Heliotropium Sclerocarpum and Evaluation of the Antioxidant Properties of the Phenolic Compounds and the Resin
Abstract
:1. Introduction
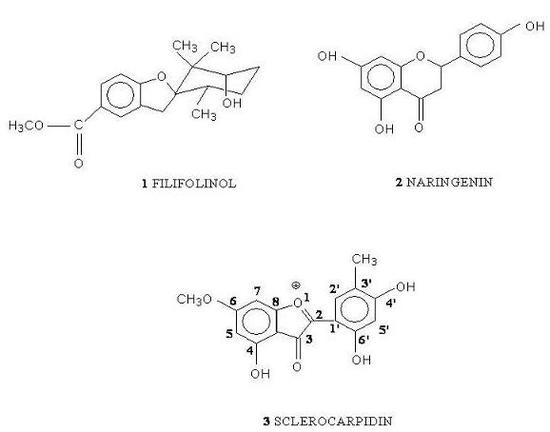
2. Results and Discussion
2.1. Analysis of the chemical composition of the resin


2.2. Antioxidant activity
| Compounds | Number of phenolic groups | FRE | TRE |
|---|---|---|---|
| 2 | 3 | 7 × 10-3 | 3.6 × 10 -2 |
| 3 | 3 | 7 × 10-4 | 8.7 × 10-4 |
| Resin | - | 5.9 × 10-2 | 18.2 × 10-2 |
| Trolox® | 1 | 1.0 | 1.0 |
| Resinous exudate | % DPPH consumed |
|---|---|
| Resin of Heliotropium sclerocarpum | 47.3 |
| Resin of Heliotropium filifolium | 44.3 |
| Resin of Heliotropium sinuatum | 48.9 |
| Resin of Heliotropium stenophyllum | 20.2 |
3. Experimental
3.1. General
3.2. Plant material
3.3. Isolation and characterization of constituent of the resin
3.4. Antioxidant activity determination
4. Conclusions
Acknowledgements
- Samples Availability: Samples of the compounds 1-3 are available from the authors.
References
- Dell, B.; Comb, J.A. Plant resins, their formation, secretion and possible functions. Adv. Bot. Res. 1978, 6, 276–316. [Google Scholar]
- Wagner, J.; Wang, E.; Shepherd, R. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef]
- Villarroel, L.; Torres, R.; Urzúa, A. Compuestos fenólicos en el exudado resinoso de Heliotropium stenophyllum. Determinación estructural y efecto antialimentario y antioxidante. Bol. Soc. Chil. Quím. 1991, 36, 169–174. [Google Scholar]
- Torres, R.; Villarroel, L.; Urzúa, A.; Delle-Monache, F.; Delle-Monache, G.; Gacs-Baitz, E. Filifolinol, a rearranged geranyl aromatic derivative from the resinous exudate of Heliotropium filifolium. Phytochemistry 1994, 36, 249–256. [Google Scholar]
- Torres, R.; Modak, B.; Urzúa, A.; Villarroel, L.; Delle-Monache, F.; Sánchez-Ferrando, F. Flavonoides del exudado resinoso de Heliotropium sinuatum. Bol. Soc. Chil. Quím. 1996, 41, 195–197. [Google Scholar]
- Urzua, A.; Modak, B.; Villarroel, L.; Torres, R.; Andrade, L. Comparative flavonoid composition of the resinous exudates from Heliotropium chenopodiaceum var. chenopodiaceum and H. chenopodiaceum var. ericoideum. Biochem. Syst. Ecol. 1998, 26, 127–130. [Google Scholar] [CrossRef]
- Urzua, A.; Modak, B.; Villarroel, L.; Torres, R.; Andrade, L.; Mendoza, L.; Wilkens, M. External flavonoids from Heliotropium megalanthum and H. huascoense (Boraginaceae). Chemotaxonomic considerations. Bol. Soc. Chil. Quím. 2000, 45, 23–29. [Google Scholar]
- Urzua, A.; Modak, B.; Torres, R. Identification of a new aromatic geranyl derivative in the resinous exudate of Heliotropium filifolium (Boraginaceae). Bol. Soc. Chil. Quím. 2001, 46, 175–178. [Google Scholar]
- Modak, B.; Torres, R.; Lissi, E.; Delle-Monache, F. Antioxidant capacity of flavonoids and a new arylphenol of the resinous exudate from Heliotropium sinuatum. Nat. Prod. Res. 2003, 17, 403–407. [Google Scholar] [CrossRef]
- Modak, B.; Rojas, M.; Torres, R.; Rodilla, J.; Luebert, F. Antioxidant activity of a new aromatic geranyl derivative of the resinous exudates from Heliotropium glutinosum Phil. Molecules 2007, 12, 1057–1063. [Google Scholar] [CrossRef]
- Modak, B.; Rojas, M.; Torres, R. Chemical Analysis of the resinous exudate isolated from Heliotropium taltalense and evaluation of the antioxidant activity of the phenolics components and the resin in homogeneous and heterogeneous systems. Molecules 2009, 14, 1980–1989. [Google Scholar] [CrossRef]
- Lissi, E.; Modak, B.; Torres, R.; Escobar, J.; Urzúa, A. Total antioxidant potential of resinous exudates from Heliotropium species, and a comparison of the ABTS and DPPH methods. Free Radic. Res. 1999, 30, 471–477. [Google Scholar] [CrossRef]
- Pannala, A.; Chan, T.; O’Brien, P.; Rice-Evans, C. Flavonoids B-ring chemistry and antioxidantactivity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R. Antioxidant and prooxidant behaviour of flavonoids: structure-ractivity. Free Radical Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Modak, B.; Contreras, M.; González-Nilo, F.; Torres, R. Structure-antioxidant activity relationships of flavonoids isolated from the resinous exudate of Heliotropium sinuatum. Bioorg. Med. Chem. Lett. 2005, 15, 309–312. [Google Scholar] [CrossRef]
- Young, J.; Hee, E.; Weon, D.; Cheon, T.; Seok, E.; Woo, E.; Gwang, H. Suppression of CYP1A1 expression by naringenin in murine Hepa-1c1c7 cells. Arch. Pharm. Res. 2004, 27, 857–862. [Google Scholar]
- Hantke, B.; Lahmann, C.; Venzke, K.; Fischer, T.; Kocourek, A.; Windsor, J.L.; Bergemann, J.; Stäb, F.; Tschesche, H. Influence of flavonoids and vitamins on the MMP- and TIMP-expression of human dermal fibroblasts after UVA irradiation. Photochem. Photobiol. Sci. 2002, 1, 826–833. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. Food Sci. Technol. Lebensm. Wiss. 1995, 28, 25–30. [Google Scholar]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents ofsome selected Iranian medicinal plants. African J.Biotech. 2006, 5, 1142–1145. [Google Scholar]
- Szabo, M.; Idit¸Oiu, C.; Chambre, D.; Lupea, A.X. Improved DPPH determination for antioxidant activity spectrophotometric assay. Chem. Pap. 2007, 61, 214–216. [Google Scholar] [CrossRef]
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Gürel, A.; Aktaş, L.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant Physiol. 2008, 34, 67–78. [Google Scholar]
- Perez, D.; Leighton, F.; Aspee, A.; Aliaga, C.; Lissi, E. A comparison of methods employed to evaluate antioxidant capabilities. Biol. Res. 2000, 33, 71–77. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Modak, B.; Salina, M.; Rodilla, J.; Torres, R. Study of the Chemical Composition of the Resinous Exudate Isolated from Heliotropium Sclerocarpum and Evaluation of the Antioxidant Properties of the Phenolic Compounds and the Resin. Molecules 2009, 14, 4625-4633. https://doi.org/10.3390/molecules14114625
Modak B, Salina M, Rodilla J, Torres R. Study of the Chemical Composition of the Resinous Exudate Isolated from Heliotropium Sclerocarpum and Evaluation of the Antioxidant Properties of the Phenolic Compounds and the Resin. Molecules. 2009; 14(11):4625-4633. https://doi.org/10.3390/molecules14114625
Chicago/Turabian StyleModak, Brenda, Melissa Salina, Jesús Rodilla, and René Torres. 2009. "Study of the Chemical Composition of the Resinous Exudate Isolated from Heliotropium Sclerocarpum and Evaluation of the Antioxidant Properties of the Phenolic Compounds and the Resin" Molecules 14, no. 11: 4625-4633. https://doi.org/10.3390/molecules14114625
APA StyleModak, B., Salina, M., Rodilla, J., & Torres, R. (2009). Study of the Chemical Composition of the Resinous Exudate Isolated from Heliotropium Sclerocarpum and Evaluation of the Antioxidant Properties of the Phenolic Compounds and the Resin. Molecules, 14(11), 4625-4633. https://doi.org/10.3390/molecules14114625