Nano and Microtechnologies for the Delivery of Oligonucleotides with Gene Silencing Properties
Abstract
:Introduction
Lipid-based Delivery Systems
Cationic liposomes
| Chemical structure | Notes |
|---|---|
 | Cationic lipid used in the formulation of cationic liposomes [23,28,31,36] |
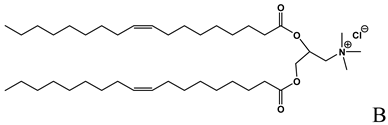 | Cationic lipid used in the formulation of cationic liposomes and LPDs [15,16, 18,19,21,22,23,24,26,27,29,35,39,41,43,44,47,48,49,56], |
 | Cationic lipid used in the formulation of cationic liposomes [14] |
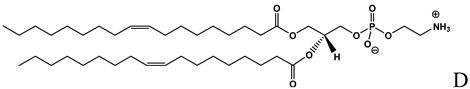 | Helper lipid with fusogenic properties used in the formulation of cationic liposomes [14,15,16, 19,20,22,24,27,30,31,38,40,42,43,] |
 | Helper lipid used in the formulation of cationic liposomes, LPDs, SNALPs and HVJ liposomes [16,18,20,27,33,36,47,48,49,50,51,52,53,55,56,57] |
 | Cationic lipid used in the formulation of cationic liposomes and LPDs [38,55] |
 | Ionizable lipid used in the formulation of cationic liposomes and SNALPs [24,50,51] |
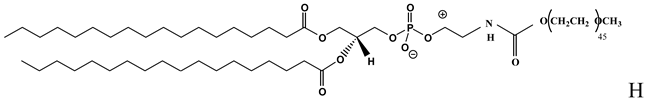 | PEGylated lipid used in the formulation of LPDs [47,48,49] |
Other lipid-based delivery systems
Polymer-based Delivery Systems
Poly(ethyleneimine)
| Chemical structure and name | Notes |
|---|---|
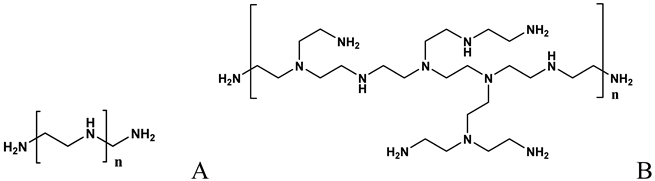 | Formation of complexes (polyplexes) by ionic interaction with ON [60,61,62,64,65,67,71,73,74,75,79] |
 | Entrapment of ON through different mechanisms, including ionic crosslinking, desolvation, or ionic complexation[76,84,85,86,87,88,89,90,91,92,93,94] |
 | Formation of complexes with ON by ionic interaction [73,98,99,100,101,102,103,104,105,106,109,110] |
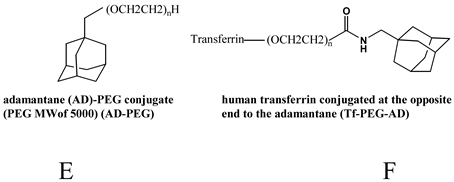 | Hydrophobic interaction with CDP [73,104,106,107,108,109,110] |
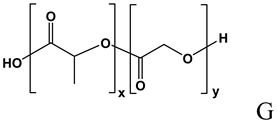 | Physical entrapment of ON [113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132] |
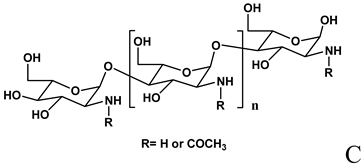
Chitosan
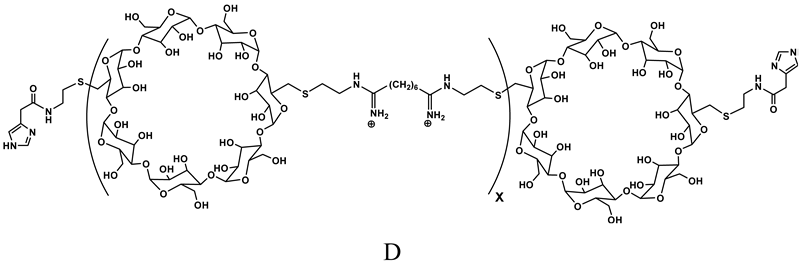
Cyclodextrin-containing polymers
Poly(lactide-co-glycolide) nano and microspheres
Conclusions
- Samples Availability: Not available.
References
- Boul-Fadl, T. Antisense oligonucleotides: the state of the art. Curr.Med.Chem. 2005, 12, 2193–2214. [Google Scholar] [CrossRef]
- Guntaka, R.V.; Varma, B.R.; Weber, K.T. Triplex-forming oligonucleotides as modulators of gene expression. Int. J. Biochem.Cell Biol. 2003, 35, 22–31. [Google Scholar] [CrossRef]
- Bielinska, A.; Shivdasani, R.A.; Zhang, L.Q.; Nabel, G.J. Regulation of gene expression with double-stranded phosphorothioate oligonucleotides. Science 1990, 250, 997–1000. [Google Scholar]
- Ulrich, H. DNA and RNA aptamers as modulators of protein function. Med. Chem. 2005, 1, 199–208. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Jorgensen, R. Altered gene expression in plants due to trans interactions between homologous genes. Trends Biotechnol. 1990, 8, 340–344. [Google Scholar] [CrossRef]
- Romano, N.; Macino, G. Quelling: transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Mol. Microbiol. 1992, 6, 3343–3353. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Lendeckel, W.; Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001, 15, 188–200. [Google Scholar] [CrossRef]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar]
- Tuschl, T.; Zamore, P.D.; Lehmann, R.; Bartel, D.P.; Sharp, P.A. Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev. 1999, 13, 3191–3197. [Google Scholar] [CrossRef]
- Behlke, M.A. Progress towards in vivo use of siRNAs. Mol. Ther. 2006, 13, 644–670. [Google Scholar] [CrossRef]
- Xie, F.Y.; Woodle, M.C.; Lu, P.Y. Harnessing in vivo siRNA delivery for drug discovery and therapeutic development. Drug Discov. Today 2006, 11, 67–73. [Google Scholar] [CrossRef]
- Stein, C.A.; Tonkinson, J.L.; Zhang, L.M.; Yakubov, L.; Gervasoni, J.; Taub, R.; Rotenberg, S.A. Dynamics of the internalization of phosphodiester oligodeoxynucleotides in HL60 cells . Biochemistry 1993, 32, 4855–4861. [Google Scholar] [CrossRef]
- De Rosa, G.; Stefano, D.D.; Laguardia, V.; Arpicco, S.; Simeon, V.; Carnuccio, R.; Fattal, E. Novel cationic liposome formulation for the delivery of an oligonucleotide decoy to NF-[kappa]B into activated macrophages. Euro. J. Pharm. Biopharm. 2008, 70, 7–18. [Google Scholar] [CrossRef]
- Jääskeläinen, I.; Sternberg, B.; Mönkkönen, J.; Urtti, A. Physicochemical and morphological properties of complexes made of cationic liposomes and oligonucleotides. Int. J. Pharm. 1998, 167, 191–203. [Google Scholar] [CrossRef]
- Jääskeläinen, I.; Peltola, S.; Honkakoski, P.; Mönkkönen, J.; Urtti, A. A lipid carrier with a membrane active component and a small complex size are required for efficient cellular delivery of anti-sense phosphorothioate oligonucleotides. Euro. J. Pharm. Sci. 2000, 10, 187–193. [Google Scholar] [CrossRef]
- Jääskeläinen, I.; Mönkkönen, J.; Urtti, A. Oligonucleotide-cationic liposome interactions. A physicochemical study. Biochim. Biophys. Acta 1994, 1195, 115–123. [Google Scholar]
- Weisman, S.; Hirsch-Lerner, D.; Barenholz, Y.; Talmon, Y. Nanostructure of cationic lipid-oligonucleotide complexes. Biophys. J. 2004, 87, 609–614. [Google Scholar] [CrossRef]
- Bouxsein, N.F.; McAllister, C.S.; Ewert, K.K.; Samuel, C.E.; Safinya, C.R. Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA. Biochemistry 2007, 46, 4785–4792. [Google Scholar] [CrossRef]
- De, R.G.; De, S.D.; Laguardia, V.; Arpicco, S.; Simeon, V.; Carnuccio, R.; Fattal, E. Novel cationic liposome formulation for the delivery of an oligonucleotide decoy to NF-kappaB into activated macrophages. Eur. J. Pharm. Biopharm. 2008, 70, 7–18. [Google Scholar] [CrossRef]
- Zelphati, O.; Uyechi, L.S.; Barron, L.G.; Szoka, F.C., Jr. Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim. Biophys. Acta 1998, 1390, 119–133. [Google Scholar]
- Yadava, P.; Gibbs, M.; Castro, C.; Hughes, J.A. Effect of lyophilization and freeze-thawing on the stability of siRNA-liposome complexes. AAPSPharm. Sci. Tech. 2008, 9, 335–341. [Google Scholar]
- Capaccioli, S.; Di, P.G.; Mini, E.; Mazzei, T.; Quattrone, A. Cationic lipids improve antisense oligonucleotide uptake and prevent degradation in cultured cells and in human serum. Biochem. Biophys. Res. Commun. 1993, 197, 818–825. [Google Scholar] [CrossRef]
- Lappalainen, K.; Urtti, A.; Soderling, E.; Jaaskelainen, I.; Syrjanen, K.; Syrjanen, S. Cationic liposomes improve stability and intracellular delivery of antisense oligonucleotides into CaSki cells. Biochim. Biophys. Acta 1994, 1196, 201–208. [Google Scholar]
- Cao, A.; Briane, D.; Coudert, R.; Vassy, J.; Lievre, N.; Olsman, E.; Tamboise, E.; Salzmann, J.L.; Rigaut, J.P.; Taillandier, E. Delivery and pathway in MCF7 cells of DNA vectorized by cationic liposomes derived from cholesterol. Antisense. Nucleic Acid Drug Dev. 2000, 10, 369–380. [Google Scholar] [CrossRef]
- Zelphati, O.; Szoka, F.C., Jr. Intracellular distribution and mechanism of delivery of oligonucleotides mediated by cationic lipids. Pharm. Res. 1996, 13, 1367–1372. [Google Scholar] [CrossRef]
- Zelphati, O.; Szoka, F.C., Jr. Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl. Acad. Sci. USA 1996, 93, 11493–11498. [Google Scholar] [CrossRef]
- Bennett, C.F.; Chiang, M.Y.; Chan, H.; Shoemaker, J.E.; Mirabelli, C.K. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol. Pharmacol. 1992, 41, 1023–1033. [Google Scholar]
- Marcusson, E.G.; Bhat, B.; Manoharan, M.; Bennett, C.F.; Dean, N.M. Phosphorothioate oligodeoxyribonucleotides dissociate from cationic lipids before entering the nucleus. Nucleic Acids Res. 1998, 26, 2016–2023. [Google Scholar] [CrossRef]
- Lavigne, C.; Thierry, A.R. Specific subcellular localization of siRNAs delivered by lipoplex in MCF-7 breast cancer cells. Biochimie 2007, 89, 1245–1251. [Google Scholar] [CrossRef]
- Bennett, C.F.; Mirejovsky, D.; Crooke, R.M.; Tsai, Y.J.; Felgner, J.; Sridhar, C.N.; Wheeler, C.J.; Felgner, P.L. Structural requirements for cationic lipid mediated phosphorothioate oligonucleotides delivery to cells in culture. J. Drug Target 1998, 5, 149–162. [Google Scholar] [CrossRef]
- Litzinger, D.C.; Huang, L. Phosphatidylethanolamine liposomes: drug delivery, gene transfer and immunodiagnostic applications. Biochim. Biophys. Acta 1992, 1113, 201–227. [Google Scholar]
- Bennett, M.J.; Nantz, M.H.; Balasubramaniam, R.P.; Gruenert, D.C.; Malone, R.W. Cholesterol enhances cationic liposome-mediated DNA transfection of human respiratory epithelial cells. Biosci. Rep. 1995, 15, 47–53. [Google Scholar] [CrossRef]
- Dass, C.R. Liposome-mediated delivery of oligodeoxynucleotides in vivo. Drug Deliv. 2002, 9, 169–180. [Google Scholar] [CrossRef]
- Miagkov, A.V.; Kovalenko, D.V.; Brown, C.E.; Didsbury, J.R.; Cogswell, J.P.; Stimpson, S.A.; Baldwin, A.S.; Makarov, S.S. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc. Natl. Acad. Sci. USA 1998, 95, 13859–13864. [Google Scholar]
- Kuramoto, Y.; Kawakami, S.; Zhou, S.; Fukuda, K.; Yamashita, F.; Hashida, M. Efficient peritoneal dissemination treatment obtained by an immunostimulatory phosphorothioate-type CpG DNA/cationic liposome complex in mice. J. Control. Release 2008, 126, 274–280. [Google Scholar] [CrossRef]
- Palliser, D.; Chowdhury, D.; Wang, Q.Y.; Lee, S.J.; Bronson, R.T.; Knipe, D.M.; Lieberman, J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature 2006, 439, 89–94. [Google Scholar]
- Litzinger, D.C.; Brown, J.M.; Wala, I.; Kaufman, S.A.; Van, G.Y.; Farrell, C.L.; Collins, D. Fate of cationic liposomes and their complex with oligonucleotide in vivo. Biochim. Biophys. Acta 1996, 1281, 139–149. [Google Scholar] [CrossRef]
- de Wolf, H.K.; Snel, C.J.; Verbaan, F.J.; Schiffelers, R.M.; Hennink, W.E.; Storm, G. Effect of cationic carriers on the pharmacokinetics and tumor localization of nucleic acids after intravenous administration. Int. J. Pharm. 2007, 331, 167–175. [Google Scholar] [CrossRef]
- Khoury, M.; Louis-Plence, P.; Escriou, V.; Noel, D.; Largeau, C.; Cantos, C.; Scherman, D.; Jorgensen, C.; Apparailly, F. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum. 2006, 54, 1867–1877. [Google Scholar] [CrossRef]
- Sorensen, D.R.; Leirdal, M.; Sioud, M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J. Mol. Biol. 2003, 327, 761–766. [Google Scholar] [CrossRef]
- Sato, A.; Takagi, M.; Shimamoto, A.; Kawakami, S.; Hashida, M. Small interfering RNA delivery to the liver by intravenous administration of galactosylated cationic liposomes in mice. Biomaterials 2007, 28, 1434–1442. [Google Scholar] [CrossRef]
- Pirollo, K.F.; Zon, G.; Rait, A.; Zhou, Q.; Yu, W.; Hogrefe, R.; Chang, E.H. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum .Gene Ther. 2006, 17, 117–124. [Google Scholar] [CrossRef]
- Ma, Z.; Li, J.; He, F.; Wilson, A.; Pitt, B.; Li, S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem. Biophys. Res. Commun. 2005, 330, 755–759. [Google Scholar] [CrossRef]
- Dritschilo, A.; Huang, C.H.; Rudin, C.M.; Marshall, J.; Collins, B.; Dul, J.L.; Zhang, C.; Kumar, D.; Gokhale, P.C.; Ahmad, A.; Ahmad, I.; Sherman, J.W.; Kasid, U.N. Phase I study of liposome-encapsulated c-raf antisense oligodeoxyribonucleotide infusion in combination with radiation therapy in patients with advanced malignancies. Clin. Cancer Res. 2006, 12, 1251–1259. [Google Scholar]
- Rudin, C.M.; Marshall, J.L.; Huang, C.H.; Kindler, H.L.; Zhang, C.; Kumar, D.; Gokhale, P.C.; Steinberg, J.; Wanaski, S.; Kasid, U.N.; Ratain, M.J. Delivery of a liposomal c-raf-1 antisense oligonucleotide by weekly bolus dosing in patients with advanced solid tumors: a phase I study. Clin. Cancer Res. 2004, 10, 7244–7251. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol. Pharm. 2006, 3, 579–588. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Surface-modified LPD nanoparticles for tumor targeting. Ann. N.Y. Acad. Sci. 2006, 1082, 1–8. [Google Scholar]
- Chono, S.; Li, S.D.; Conwell, C.C.; Huang, L. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. J. Control. Release 2008, 131, 64–69. [Google Scholar] [CrossRef]
- Semple, S. C.; Klimuk, S. K.; Harasym, T. O.; Dos, S. N.; Ansell, S. M.; Wong, K. F.; Maurer, N.; Stark, H.; Cullis, P. R.; Hope, M. J.; Scherrer, P. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta 2001, 1510, 152–166. [Google Scholar] [CrossRef]
- Leonetti, C.; Biroccio, A.; Benassi, B.; Stringaro, A.; Stoppacciaro, A.; Semple, S.C.; Zupi, G. Encapsulation of c-myc antisense oligodeoxynucleotides in lipid particles improves antitumoral efficacy in vivo in a human melanoma line. Cancer. Gene Ther. 2001, 8, 459–468. [Google Scholar] [CrossRef]
- Morrissey, D.V.; Lockridge, J.A.; Shaw, L.; Blanchard, K.; Jensen, K.; Breen, W.; Hartsough, K.; Machemer, L.; Radka, S.; Jadhav, V.; Vaish, N.; Zinnen, S.; Vargeese, C.; Bowman, K.; Shaffer, C. S.; Jeffs, L.B.; Judge, A.; MacLachlan, I.; Polisky, B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005, 23, 1002–1007. [Google Scholar] [CrossRef]
- Zimmermann, T.S.; Lee, A.C.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harborth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; Judge, A.D.; Lam, K.; McClintock, K.; Nechev, L.V.; Palmer, L.R.; Racie, T.; Rohl, I.; Seiffert, S.; Shanmugam, S.; Sood, V.; Soutschek, J.; Toudjarska, I.; Wheat, A.J.; Yaworski, E.; Zedalis, W.; Koteliansky, V.; Manoharan, M.; Vornlocher, H.P.; MacLachlan, I. RNAi-mediated gene silencing in non-human primates. Nature 2006, 441, 111–114. [Google Scholar]
- Kaneda, Y. New vector innovation for drug delivery: development of fusigenic non-viral particles . Curr. Drug Targets 2003, 4, 599–602. [Google Scholar] [CrossRef]
- Nakamura, N.; Hart, D.A.; Frank, C.B.; Marchuk, L.L.; Shrive, N.G.; Ota, N.; Taira, K.; Yoshikawa, H.; Kaneda, Y. Efficient transfer of intact oligonucleotides into the nucleus of ligament scar fibroblasts by HVJ-cationic liposomes is correlated with effective antisense gene inhibition. J. Biochem. 2001, 129, 755–759. [Google Scholar] [CrossRef]
- Takeuchi, K.; Itoh, H.; Yonemitsu, Y.; Matsumoto, T.; Kume, M.; Komori, K.; Maehara, Y. In vivo reduction of the nuclear factor-kappaB activity using synthetic cis-element decoy oligonucleotides suppresses intimal hyperplasia in the injured carotid arteries in rabbits. Surg.Today 2007, 37, 575–583. [Google Scholar] [CrossRef]
- Omae, T.; Yoshioka, H.; Tanaka, T.; Nagai, H.; Saji, M.; Noda, K.; Kobayashi, S.; Sugimoto, T. Antisense in vivo knockdown of synaptotagmin I by HVJ-liposome mediated gene transfer attenuates ischemic brain damage in neonatal rats. Brain Dev. 2008, 30, 313–320. [Google Scholar] [CrossRef]
- Son, G.; Iimuro, Y.; Seki, E.; Hirano, T.; Kaneda, Y.; Fujimoto, J. Selective inactivation of NF-kappaB in the liver using NF-kappaB decoy suppresses CCl4-induced liver injury and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G631–G639. [Google Scholar] [CrossRef]
- von, H.A.; Petersen, H.; Li, Y.; Kissel, T. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J. Control. Release 2000, 69, 309–322. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc'h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar]
- Gomes dos Santos, A.L.; Bochot, A.; Tsapis, N.; Artzner, F.; Bejjani, R.A.; Thillaye-Goldenberg, B.; de, K.Y.; Fattal, E.; Behar-Cohen, F. Oligonucleotide-polyethylenimine complexes targeting retinal cells: structural analysis and application to anti-TGFbeta-2 therapy. Pharm. Res. 2006, 23, 770–781. [Google Scholar] [CrossRef]
- Grayson, A.C.; Doody, A.M.; Putnam, D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm. Res. 2006, 23, 1868–1876. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Bronich, T.K.; Kabanov, A.V. Self-assembly of polyamine-poly(ethylene glycol) copolymers with phosphorothioate oligonucleotides. Bioconjug. Chem. 1998, 9, 805–812. [Google Scholar] [CrossRef]
- Glodde, M.; Sirsi, S.R.; Lutz, G.J. Physiochemical properties of low and high molecular weight poly(ethylene glycol)-grafted poly(ethylene imine) copolymers and their complexes with oligonucleotides. Biomacromolecules 2006, 7, 347–356. [Google Scholar] [CrossRef]
- Mao, S.; Neu, M.; Germershaus, O.; Merkel, O.; Sitterberg, J.; Bakowsky, U.; Kissel, T. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjug. Chem. 2006, 17, 1209–1218. [Google Scholar] [CrossRef]
- Brus, C.; Petersen, H.; Aigner, A.; Czubayko, F.; Kissel, T. Efficiency of polyethylenimines and polyethylenimine-graft-poly (ethylene glycol) block copolymers to protect oligonucleotides against enzymatic degradation. Eur. J. Pharm. Biopharm. 2004, 57, 427–430. [Google Scholar] [CrossRef]
- Remaut, K.; Lucas, B.; Raemdonck, K.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Protection of oligonucleotides against enzymatic degradation by pegylated and nonpegylated branched polyethyleneimine. Biomacromolecules 2007, 8, 1333–1340. [Google Scholar] [CrossRef]
- Brus, C.; Santi, P.; Colombo, P.; Kissel, T. Distribution and quantification of polyethylenimine oligodeoxynucleotide complexes in human skin after iontophoretic delivery using confocal scanning laser microscopy. J.Control. Release 2002, 84, 171–181. [Google Scholar] [CrossRef]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine) and its role in gene delivery. J. Control. Release 1999, 60, 149–160. [Google Scholar] [CrossRef]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc. Natl. Acad. Sci. USA 1999, 96, 5177–5181. [Google Scholar] [CrossRef]
- Sirsi, S.R.; Williams, J.H.; Lutz, G.J. Poly(ethylene imine)-poly(ethylene glycol) copolymers facilitate efficient delivery of antisense oligonucleotides to nuclei of mature muscle cells of mdx mice. Hum.Gene Ther. 2005, 16, 1307–1317. [Google Scholar] [CrossRef]
- Brus, C.; Petersen, H.; Aigner, A.; Czubayko, F.; Kissel, T. Physicochemical and biological characterization of polyethylenimine-graft-poly(ethylene glycol) block copolymers as a delivery system for oligonucleotides and ribozymes. Bioconjug. Chem. 2004, 15, 677–684. [Google Scholar] [CrossRef]
- Kulkarni, R.P.; Mishra, S.; Fraser, S.E.; Davis, M.E. Single cell kinetics of intracellular, nonviral, nucleic acid delivery vehicle acidification and trafficking. Bioconjug. Chem. 2005, 16, 986–994. [Google Scholar] [CrossRef]
- Werth, S.; Urban-Klein, B.; Dai, L.; Hobel, S.; Grzelinski, M.; Bakowsky, U.; Czubayko, F.; Aigner, A. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes . J. Control. Release 2006, 112, 257–270. [Google Scholar] [CrossRef]
- Fischer, D.; Osburg, B.; Petersen, H.; Kissel, T.; Bickel, U. Effect of poly(ethylene imine) molecular weight and pegylation on organ distribution and pharmacokinetics of polyplexes with oligodeoxynucleotides in mice. Drug Metab. Dispos. 2004, 32, 983–992. [Google Scholar]
- Howard, K.A.; Rahbek, U.L.; Liu, X.; Damgaard, C.K.; Glud, S.Z.; Andersen, M.O.; Hovgaard, M.B.; Schmitz, A.; Nyengaard, J.R.; Besenbacher, F.; Kjems, J. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol. Ther. 2006, 14, 476–484. [Google Scholar] [CrossRef]
- Weerasinghe, P.; Li, Y.; Guan, Y.; Zhang, R.; Tweardy, D.J.; Jing, N. T40214/PEI complex: A potent therapeutics for prostate cancer that targets STAT3 signaling. Prostate 2008, 68, 1430–1442. [Google Scholar] [CrossRef]
- Urban-Klein, B.; Werth, S.; Abuharbeid, S.; Czubayko, F.; Aigner, A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005, 12, 461–466. [Google Scholar] [CrossRef]
- Bonnet, M.E.; Erbacher, P.; Bolcato-Bellemin, A.L. Systemic delivery of DNA or siRNA mediated by linear polyethylenimine (L-PEI) does not induce an inflammatory response. Pharm. Res. 2008, 25, 2972–2982. [Google Scholar] [CrossRef]
- Jiang, G.; Park, K.; Kim, J.; Kim, K.S.; Hahn, S.K. Target Specific Intracellular Delivery of siRNA/PEI-HA Complex by Receptor Mediated Endocytosis. Mol. Pharm. 2009, 6, 727–737. [Google Scholar] [CrossRef]
- Sirsi, S.R.; Schray, R.C.; Guan, X.; Lykens, N.M.; Williams, J.H.; Erney, M.L.; Lutz, G.J. Functionalized PEG-PEI copolymers complexed to exon-skipping oligonucleotides improve dystrophin expression in mdx mice. Hum. Gene Ther. 2008, 19, 795–806. [Google Scholar] [CrossRef]
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Singla, A.K.; Chawla, M. Chitosan: some pharmaceutical and biological aspects--an update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef]
- Katas, H.; Alpar, H.O. Development and characterisation of chitosan nanoparticles for siRNA delivery. J. Control. Release 2006, 115, 216–225. [Google Scholar] [CrossRef]
- Liu, X.; Howard, K.A.; Dong, M.; Andersen, M.O.; Rahbek, U.L.; Johnsen, M.G.; Hansen, O.C.; Besenbacher, F.; Kjems, J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 2007, 28, 1280–1288. [Google Scholar] [CrossRef]
- Dung, T.H.; Lee, S.R.; Han, S.D.; Kim, S.J.; Ju, Y.M.; Kim, M.S.; Yoo, H. Chitosan-TPP nanoparticle as a release system of antisense oligonucleotide in the oral environment. J. Nanosci. Nanotechnol. 2007, 7, 3695–3699. [Google Scholar] [CrossRef]
- Kim, S.T.; Kim, C.K. Water-soluble chitosan-based antisense oligodeoxynucleotide of interleukin-5 for treatment of allergic rhinitis . Biomaterials 2007, 28, 3360–3368. [Google Scholar] [CrossRef]
- Andersen, M.O.; Howard, K.A.; Paludan, S.R.; Besenbacher, F.; Kjems, J. Delivery of siRNA from lyophilized polymeric surfaces. Biomaterials 2008, 29, 506–512. [Google Scholar] [CrossRef]
- Gao, S.; Chen, J.; Dong, L.; Ding, Z.; Yang, Y.H.; Zhang, J. Targeting delivery of oligonucleotide and plasmid DNA to hepatocyte via galactosylated chitosan vector. Eur. J. Pharm. Biopharm. 2005, 60, 327–334. [Google Scholar] [CrossRef]
- Gao, S.; gnaes-Hansen, F.; Nielsen, E. J. B.; Wengel, J.; Besenbacher, F.; Howard, K. A.; Kjems, J. The Effect of Chemical Modification and Nanoparticle Formulation on Stability and Biodistribution of siRNA in Mice. Mol. Ther. 2009, 17, 1225–1233. [Google Scholar] [CrossRef]
- Dong, L.; Gao, S.; Diao, H.; Chen, J.; Zhang, J. Galactosylated low molecular weight chitosan as a carrier delivering oligonucleotides to Kupffer cells instead of hepatocytes in vivo. J. Biomed. Mater. Res. A 2008, 84, 777–784. [Google Scholar]
- Dong, L.; Zuo, L.; Xia, S.; Gao, S.; Zhang, C.; Chen, J.; Zhang, J. Reduction of liver tumor necrosis factor-alpha expression by targeting delivery of antisense oligonucleotides into Kupffer cells protects rats from fulminant hepatitis. J. Gene Med. 2009, 11, 229–239. [Google Scholar] [CrossRef]
- Howard, K.A.; Paludan, S.R.; Behlke, M.A.; Besenbacher, F.; Deleuran, B.; Kjems, J. Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol. Ther. 2009, 17, 162–168. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Mohapatra, S.; Kong, X.; Li, X.; Lockey, R.F.; Mohapatra, S.S. Prevention of airway inflammation with topical cream containing imiquimod and small interfering RNA for natriuretic peptide receptor . Genet. Vaccines Ther. 2008, 6, 7. [Google Scholar] [CrossRef]
- Glud, S.Z.; Bramsen, J.B.; gnaes-Hansen, F.; Wengel, J.; Howard, K.A.; Nyengaard, J.R.; Kjems, J. Naked siLNA-mediated gene silencing of lung bronchoepithelium EGFP expression after intravenous administration. Oligonucleotides 2009, 19, 163–168. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Davis, M.E.; Pun, S.H.; Bellocq, N.C.; Reineke, T.M.; Popielarski, S.R.; Mishra, S.; Heidel, J.D. Self-assembling nucleic acid delivery vehicles via linear, water-soluble, cyclodextrin-containing polymers. Curr. Med. Chem. 2004, 11, 179–197. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hwang, S.J.; Davis, M.E. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug. Chem. 1999, 10, 1068–1074. [Google Scholar] [CrossRef]
- Hwang, S.J.; Bellocq, N.C.; Davis, M.E. Effects of structure of beta-cyclodextrin-containing polymers on gene delivery. Bioconjug. Chem. 2001, 12, 280–290. [Google Scholar] [CrossRef]
- Popielarski, S.R.; Mishra, S.; Davis, M.E. Structural effects of carbohydrate-containing polycations on gene delivery. 3. Cyclodextrin type and functionalization . Bioconjug. Chem. 2003, 14, 672–678. [Google Scholar] [CrossRef]
- Reineke, T.M.; Davis, M.E. Structural effects of carbohydrate-containing polycations on gene delivery. 1. Carbohydrate size and its distance from charge centers. Bioconjug. Chem. 2003, 14, 247–254. [Google Scholar] [CrossRef]
- Reineke, T.M.; Davis, M.E. Structural effects of carbohydrate-containing polycations on gene delivery. 2. Charge center type. Bioconjug. Chem. 2003, 14, 255–261. [Google Scholar] [CrossRef]
- Davis, M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef]
- Mishra, S.; Heidel, J.D.; Webster, P.; Davis, M.E. Imidazole groups on a linear, cyclodextrin-containing polycation produce enhanced gene delivery via multiple processes. J. Control. Release 2006, 116, 179–191. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Davis, M.E. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjug. Chem. 2007, 18, 456–468. [Google Scholar] [CrossRef]
- Bellocq, N.C.; Pun, S.H.; Jensen, G.S.; Davis, M.E. Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug. Chem. 2003, 14, 1122–1132. [Google Scholar] [CrossRef]
- Pun, S.H.; Davis, M.E. Development of a nonviral gene delivery vehicle for systemic application. Bioconjug. Chem. 2002, 13, 630–639. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Su, H.; Hildebrandt, I.J.; Weber, W.A.; Davis, M.E. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 15549–15554. [Google Scholar]
- Heidel, J.D.; Yu, Z.; Liu, J.Y.; Rele, S.M.; Liang, Y.; Zeidan, R.K.; Kornbrust, D.J.; Davis, M.E. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc. Natl. Acad. Sci. USA 2007, 104, 5715–5721. [Google Scholar]
- Sinha, V.R.; Trehan, A. Biodegradable microspheres for parenteral delivery. Crit. Rev. Ther. Drug Carrier Syst. 2005, 22, 535–602. [Google Scholar] [CrossRef]
- Vert, M.; Mauduit, J.; Li, S. Biodegradation of PLA/GA polymers: increasing complexity. Biomaterials 1994, 15, 1209–1213. [Google Scholar] [CrossRef]
- Lewis, K.J.; Irwin, W.J.; Akhtar, S. Biodegradable poly(-lactic acid) matrices for the sustained delivery of antisense oligonucleotides. J. Control. Release 1995, 37, 173–183. [Google Scholar] [CrossRef]
- De, R. G.; Quaglia, F.; Bochot, A.; Ungaro, F.; Fattal, E. Long-term release and improved intracellular penetration of oligonucleotide-polyethylenimine complexes entrapped in biodegradable microspheres. Biomacromolecules 2003, 4, 529–536. [Google Scholar] [CrossRef]
- Lewis, K J.; Irwin, W.J.; Akhtar, S. Development of a sustained-release biodegradable polymer delivery system for site-specific delivery of oligonucleotides: characterization of P(LA-GA) copolymer microspheres in vitro. J. Drug Target 1998, 5, 291–302. [Google Scholar] [CrossRef]
- De, R.G.; Quaglia, F.; La Rotonda, M.I.; Appel, M.; Alphandary, H.; Fattal, E. Poly(lactide-co-glycolide) microspheres for the controlled release of oligonucleotide/polyethylenimine complexes. J. Pharm. Sci. 2002, 91, 790–799. [Google Scholar]
- De, R.G.; Maiuri, M.C.; Ungaro, F.; De, S.D.; Quaglia, F.; La Rotonda, M.I.; Carnuccio, R. Enhanced intracellular uptake and inhibition of NF-kappaB activation by decoy oligonucleotide released from PLGA microspheres. J. Gene Med. 2005, 7, 771–781. [Google Scholar] [CrossRef]
- Freytag, T.; Dashevsky, A.; Tillman, L.; Hardee, G.E.; Bodmeier, R. Improvement of the encapsulation efficiency of oligonucleotide-containing biodegradable microspheres. J. Control Release 2000, 69, 197–207. [Google Scholar] [CrossRef]
- De, R.G.; Bochot, A.; Quaglia, F.; Besnard, M.; Fattal, E. A new delivery system for antisense therapy: PLGA microspheres encapsulating oligonucleotide/polyethyleneimine solid complexes. Int. J. Pharm. 2003, 254, 89–93. [Google Scholar] [CrossRef]
- Sirsi, S.R.; Schray, R.C.; Wheatley, M.A.; Lutz, G.J. Formulation of polylactide-co-glycolic acid nanospheres for encapsulation and sustained release of poly(ethylene imine)-poly(ethylene glycol) copolymers complexed to oligonucleotides. J. Nanobiotechnol. 2009, 7, 1. [Google Scholar] [CrossRef]
- Akhtar, S.; Lewis, K.J. Antisense oligonucleotide delivery to cultured macrophages is improved by incorporation into sustained-release biodegradable polymer microspheres. Int. J. Pharm. 1997, 151, 57–67. [Google Scholar] [CrossRef]
- Aukunuru, J.V.; Ayalasomayajula, S.P.; Kompella, U.B. Nanoparticle formulation enhances the delivery and activity of a vascular endothelial growth factor antisense oligonucleotide in human retinal pigment epithelial cells. J. Pharm. Pharmacol. 2003, 55, 1199–1206. [Google Scholar] [CrossRef]
- Berton, M.; Benimetskaya, L.; Allemann, E.; Stein, C.A.; Gurny, R. Uptake of oligonucleotide-loaded nanoparticles in prostatic cancer cells and their intracellular localization. Eur. J. Pharm. Biopharm. 1999, 47, 119–123. [Google Scholar] [CrossRef]
- Cleek, R.L.; Rege, A.A.; Denner, L.A.; Eskin, S.G.; Mikos, A.G. Inhibition of smooth muscle cell growth in vitro by an antisense oligodeoxynucleotide released from poly(DL-lactic-co-glycolic acid) microparticles. J. Biomed. Mater. Res. 1997, 35, 525–530. [Google Scholar] [CrossRef]
- Cohen-Sacks, H.; Najajreh, Y.; Tchaikovski, V.; Gao, G.; Elazer, V.; Dahan, R.; Gati, I.; Kanaan, M.; Waltenberger, J.; Golomb, G. Novel PDGFbetaR antisense encapsulated in polymeric nanospheres for the treatment of restenosis. Gene Ther. 2002, 9, 1607–1616. [Google Scholar] [CrossRef]
- Ungaro, F.; De Rosa, G.; Quaglia, F.; Fattal, E.; La Rotonda, M.I. Controlled release of oligonucleotide/polyethylenimine complexes from PLGA-based microspheres: potential of spray-drying technique. J. Drug Deliv. Sci. Technol. 2005, 15, 113–192. [Google Scholar]
- Lebedeva, I.; Benimetskaya, L.; Stein, C.A.; Vilenchik, M. Cellular delivery of antisense oligonucleotides. Eur. J. Pharm. Biopharm. 2000, 50, 101–119. [Google Scholar] [CrossRef]
- Khan, A.; Sommer, W.; Fuxe, K.; Akhtar, S. Site-specific administration of antisense oligonucleotides using biodegradable polymer microspheres provides sustained delivery and improved subcellular biodistribution in the neostriatum of the rat brain. J. Drug Target. 2000, 8, 319–334. [Google Scholar] [CrossRef]
- Putney, S.D.; Brown, J.; Cucco, C.; Lee, R.; Skorski, T.; Leonetti, C.; Geiser, T.; Calabretta, B.; Zupi, G.; Zon, G. Enhanced anti-tumor effects with microencapsulated c-myc antisense oligonucleotide. Antisense Nucleic Acid Drug Dev. 1999, 9, 451–458. [Google Scholar] [CrossRef]
- Diwan, M.; Elamanchili, P.; Lane, H.; Gainer, A.; Samuel, J. Biodegradable nanoparticle mediated antigen delivery to human cord blood derived dendritic cells for induction of primary T cell responses. J. Drug Target. 2003, 11, 495–507. [Google Scholar] [CrossRef]
- De, S.D.; De, R.G.; Maiuri, M.C.; Ungaro, F.; Quaglia, F.; Iuvone, T.; Cinelli, M.P.; La Rotonda, M.I.; Carnuccio, R. Oligonucleotide decoy to NF-kappaB slowly released from PLGA microspheres reduces chronic inflammation in rat. Pharmacol. Res. 2009, 60, 33–40. [Google Scholar] [CrossRef]
- Gomes dos Santos, A.L.; Bochot, A.; Doyle, A.; Tsapis, N.; Siepmann, J.; Siepmann, F.; Schmaler, J.; Besnard, M.; Behar-Cohen, F.; Fattal, E. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery. J. Control Release. 2006, 112, 369–381. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Rosa, G.; La Rotonda, M.I. Nano and Microtechnologies for the Delivery of Oligonucleotides with Gene Silencing Properties. Molecules 2009, 14, 2801-2823. https://doi.org/10.3390/molecules14082801
De Rosa G, La Rotonda MI. Nano and Microtechnologies for the Delivery of Oligonucleotides with Gene Silencing Properties. Molecules. 2009; 14(8):2801-2823. https://doi.org/10.3390/molecules14082801
Chicago/Turabian StyleDe Rosa, Giuseppe, and Maria Immacolata La Rotonda. 2009. "Nano and Microtechnologies for the Delivery of Oligonucleotides with Gene Silencing Properties" Molecules 14, no. 8: 2801-2823. https://doi.org/10.3390/molecules14082801





