Catalytic Epoxidation of a Technical Mixture of Methyl Oleate and Methyl Linoleate in Ionic Liquids Using MoO(O2)2•2QOH (QOH = 8-quinilinol) as Catalyst and NaHCO3 as co-Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst preparation
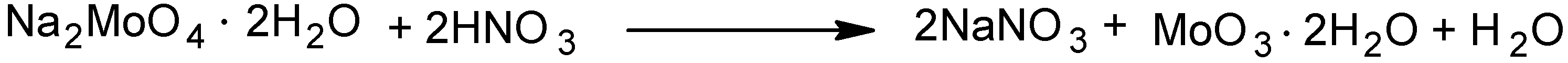
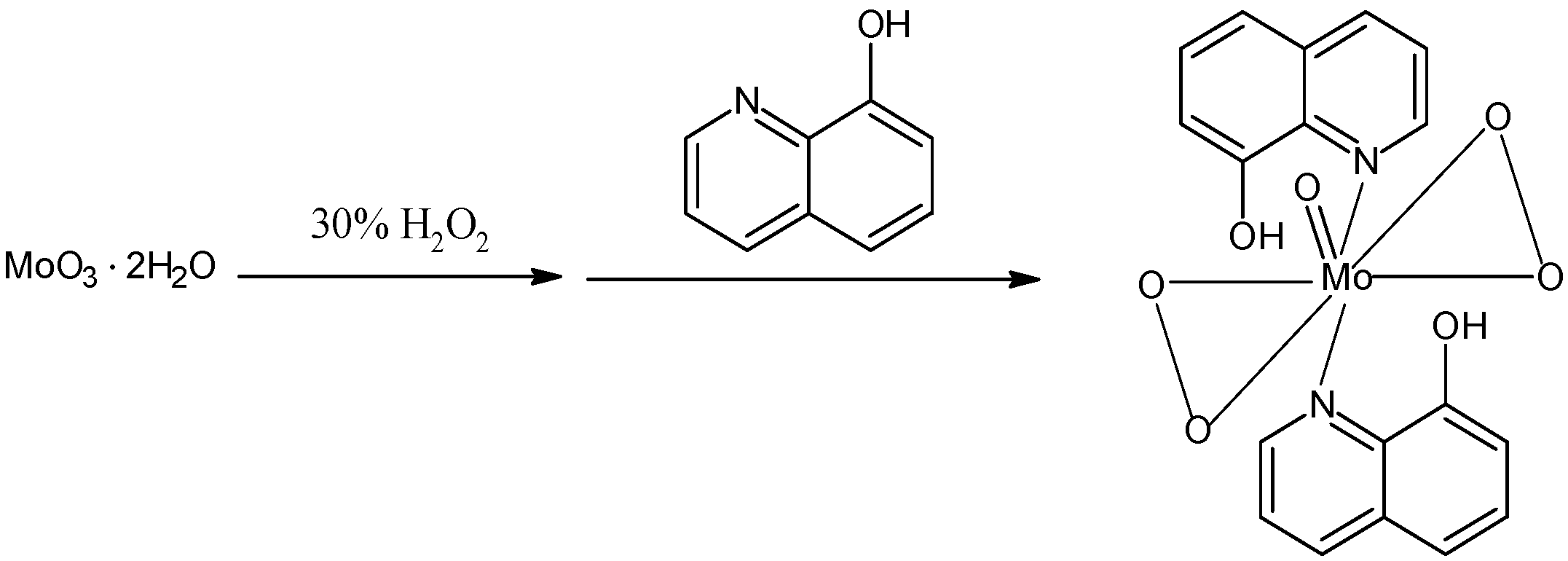
2.2. Catalytic performance
2.2.1. UV-Vis spectral study of [MoO(O2)2·2QOH] (QOH = 8-quinilinol) alone
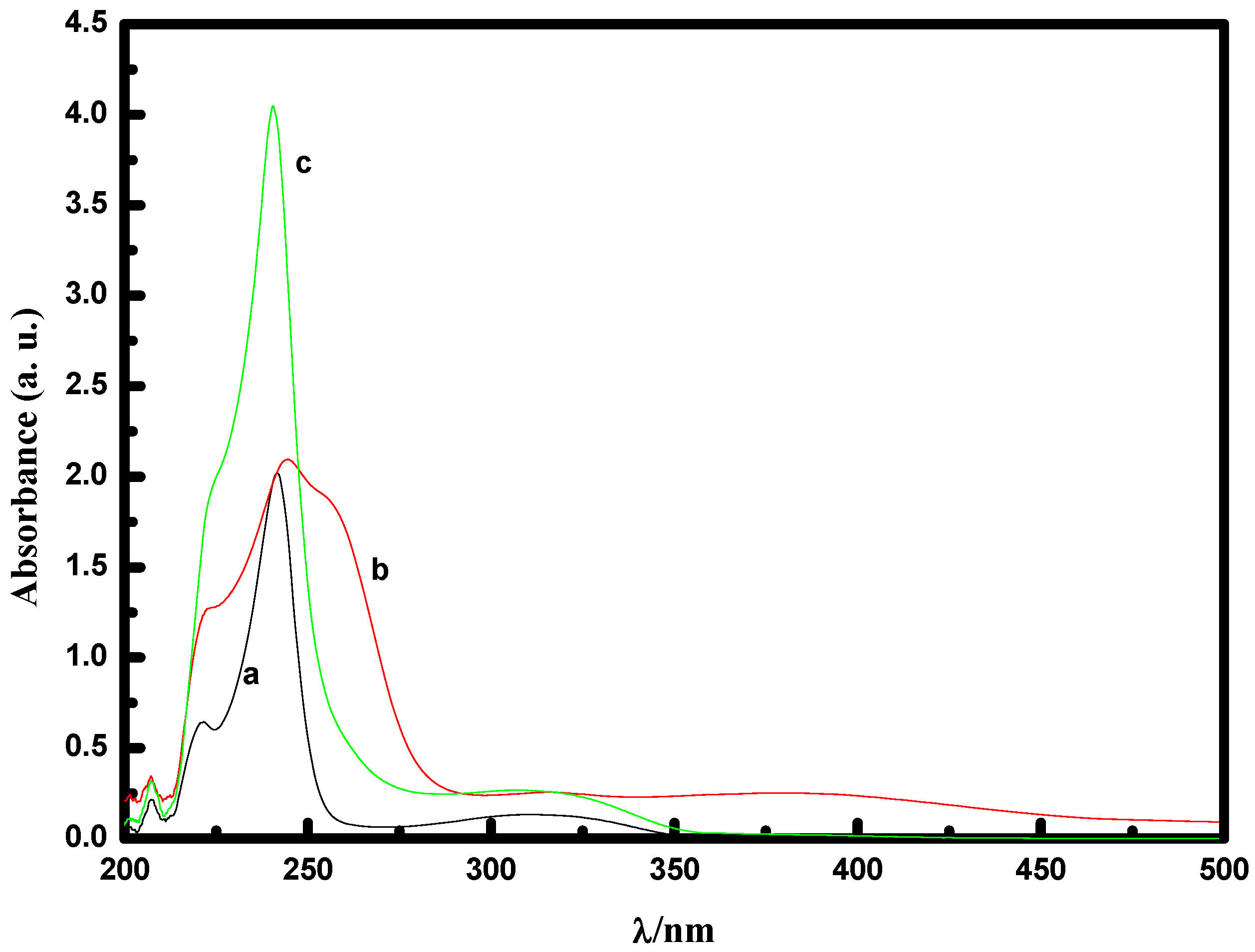
2.2.2. Effect of NaHCO3 as a co-catalyst
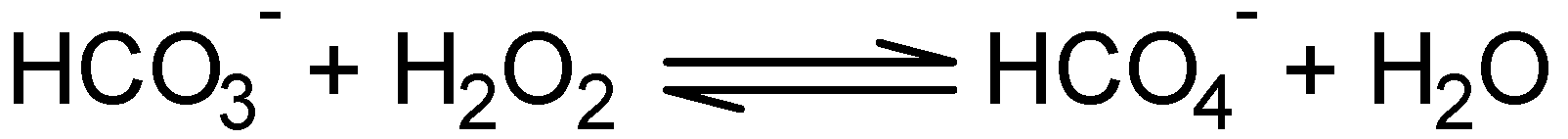
| Entry | Solvent | Conversion (%) | Selectivity (%) e | TON(TOF) f | ||
| methyl oleate b | methyl linoleate c | total d | ||||
| 1 | no solvent | 55 | 31 | 41 | 90 | 3690(1845) |
| 2 | no catalyst | 0 | 0 | 0 | 0 | 0 |
| 3 | [bmim]BF4 | 92 | 78 | 84 | 93 | 7812(3906) |
| 4 | [bmim]PF6 | 75 | 44 | 57 | 94 | 5358(2679) |
| 5 | [Hydemim]BF4 | 96 | 89 | 92 | 95 | 8740(4370) |
| 6 | [bmim]PF6g | 60 | 32 | 44 | 92 | 4048(2024) |
| 7 | [Hydemim]BF4g | 84 | 54 | 67 | 94 | 6298(3149) |
| 8 | CH3CN | 85 | 63 | 72 | 92 | 6624(3312) |
| 9 | 30% CH3CN + 70% [hydemim]BF4 | 94 | 84 | 88 | 95 | 8360(4180) |
| 10 | C2H5OH | 81 | 45 | 60 | 93 | 5580(2790) |
| 11 | 30 % C2H5OH + 70% [hydemim]BF4 | 90 | 74 | 81 | 95 | 7695(3848) |
2.2.3. Effect of ILs
2.3. Recycling of the catalyst
| Run | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Conversion (%) | methyl oleate b | 96 | 92 | 89 | 89 | 87 |
| methyl linoleate c | 89 | 87 | 86 | 82 | 82 | |
| total d | 92 | 89 | 87 | 85 | 84 | |
| Selectivity (%) e | 95 | 95 | 95 | 95 | 95 | |
3. Experimental
3.1. Materials
3.2. Instruments
3.3. Preparation
3.3.1. Preparation and characterization of MoO3·2H2O
3.3.2. Preparation and characterization of [MoO(O2)2·2QOH] (QOH = 8-quinilinol)
3.3.3. Characterization of the ILs [bmim][BF4], [hydemim][BF4], and [bmim][PF6]
3.4. Epoxidation
4. Conclusions
Appendix: Analysis of products
Determination of the conversion of methyl oleate or methyl linoleate
Determination of the total selectivity of oxidation products to epoxidized methyl oleate and methyl linoleate
References
- Gunstone, F.D. Lipid Technologies and Applications; Gunstone, F.D., Padley, F.B., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 759–769. [Google Scholar]
- Petrovic, Z.S.; Zlatanic, A.; Lava, C.C.; Sinadinovic-Fiser, S. Epoxidation of soybean oil in toluene with peroxoacetic and peroxoformic acids-kinetics and side reactions. Eur. J. Lipid Sci. Technol. 2002, 104, 293–299. [Google Scholar] [CrossRef]
- Lie Ken Jie, M.S.F.; Khysar Pasha, M. Epoxidation reactions of unsaturated fatty esters with potassium peroxomonosulfate. Lipids 1998, 33, 633–637. [Google Scholar] [CrossRef]
- Cheung, W.H.; Yu, W.Y.; Yip, W.P.; Zhu, N. Y.; Che, C.M. A silica gel-supported ruthenium complex of 1,4,7-trimethyl-1,4,7-triazacyclononane as recyclable catalyst for chemoselective oxidation of alcohols and alkenes by tert-butyl hydroperoxide. J. Org. Chem. 2002, 67, 7716–7723. [Google Scholar] [CrossRef]
- Warwel, S.; Klaas, M.R. Chemo-enzymatic epoxidation of unsaturated carboxylic acids. J. Mol. Catal. B: Enz. 1995, 1, 29–35. [Google Scholar] [CrossRef]
- Sobczak, J.M.; Ziolkowski, J.J. Molybdenum complex-catalysed epoxidation of unsaturated fatty acids by organic hydroperoxides. Appl. Catal. A-Gen. 2003, 248, 261–268. [Google Scholar] [CrossRef]
- Notari, B. Microporous crystalline titanium silicates. Adv. Catal. 1996, 41, 253–334. [Google Scholar] [CrossRef]
- Sato, K.; Aoki, M.; Ogawa, M.; Hashimaoto, T.; Panyella, D.; Noyori, R. A halide-free method for olefin epoxidation with 30 % hydrogen peroxide. Bull. Chem. Soc. Jpn. 1997, 70, 905–915. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Kratzer, R.M.; Ding, H.; Thiel, W.R.; Glas, H. Methyltrioxorhenium/pyrazole —A highly efficient catalyst for the epoxidation of olefins. J. Organomet. Chem. 1998, 555, 293–295. [Google Scholar] [CrossRef]
- Dickman, M.H.; Michael, T.R. Peroxo and superoxo complexes of chromium, molybdemum and tungsten. Chem. Rev. 1994, 94, 569–584. [Google Scholar] [CrossRef]
- Ramos, M.L.; Calderia, M.M.; Gil, V.M.S. Peroxo complexes of sugar acids with oxoions of MoVI and WVI as studied by NMR spectroscopy. J. Chem. Soc. Dalton Trans. 2000, 2099–2103. [Google Scholar] [CrossRef]
- Mimoun, H. Metal complexes in oxidation. In Comprehensive Co-ordination Chemistry; Wilkinson, G., Gillard, R.D., McCleverty, J.A., Eds.; Pergamon Press: Oxford, UK, 1987; Vol. 6, pp. 317–410. [Google Scholar]
- Ballistreri, F.P; Bazzo, A.; Tomaselli, G.A.; Toscano, R.M. Reactivity of peroxopolyoxo complexes. Oxidation of thioethers, alkenes, and sulfoxides by tetrahexylammonium tetrakis (diperoxomolybdo) phosphate. J. Org. Chem. 1992, 57, 7074–7077. [Google Scholar] [CrossRef]
- Trost, M.K.; Bergman, R.G. The Cp*MoO2Cl catalyzed epoxidation of olefins by alkyl hydroperoxides. Organometallics 1991, 10, 1172–1178. [Google Scholar] [CrossRef]
- Queeney, K.T.; Chen, D.A.; Friend, C.M. Probing the role of oxygen coordination in hydrocarbon oxidation: Methyl radical addition to oxygen on Mo(110). J. Am. Chem. Soc. 1997, 119, 6945–6946. [Google Scholar] [CrossRef]
- Maiti, S.K.; Dinda, S.; Nandi, M.; Bhaumik, A.; Bhattacharyya, R. Selective epoxidation of olefins catalyzed by oxodiperoxomolybdenum(VI) complexes immobilized over highly ordered 2D-hexagonal mesoporous silica. J. Mol. Catal. A Chem. 2008, 287, 135–141. [Google Scholar] [CrossRef]
- Noyori, R.; Aoki, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 2003, 16, 1977–1986. [Google Scholar] [CrossRef]
- Suarez, P.A.Z.; Dullius, J.E.L.; Einloft, S.; de Souza, R.F.; Dupont, J. The use of new ionic liquids in two-phase catalytic hydrogenation reaction by rhodium complexes. Polyhedron 1996, 15, 1217–1219. [Google Scholar] [CrossRef]
- Dupont, J.; Suarez, P.A.Z.; de Souza, R.F.; Burrow, R.A.; Kintzinger, J.P. C-H-π interactions in 1-n-Butyl-3-methylimidazolium tetraphenylborate molten salt: Solid and solution structures. Chem. Eur. J. 2000, 6, 2377–2381. [Google Scholar] [CrossRef]
- Chauvin, Y.; Mussmann, L.; Olivier, H. A novel class of versatile solvents for two-phase catalysis: Hydrogenation, isomerization, and hydroformylation of alkenes catalyzed by rhodium complexes in liquid 1,3-dialkylimidazolium salts. Angew. Chem. Int. Ed. Engl. 1996, 34, 2698–2700. [Google Scholar] [CrossRef]
- Suarez, P.A.Z.; Selbach, V.M.; Dullius, J.E.L.; Einloft, S.; Piatnick, C.M.S.; Azambuja, D.S.; de Souza, R.F.; Dupont, J. Enlarged electrochemical window in dialkyl-imidazolium cation based room-temperature air and water-stable molten salts. Electrochim. Acta 1997, 42, 2533–2535. [Google Scholar] [CrossRef]
- Stark, A.; MacLean, B.L.; Singer, R.D. 1-Ethyl-3-methylimidazolium halogenoaluminate ionic liquids as solvents for Friedel-Crafts acylation reactions of ferrocene. J. Chem. Soc., Dalton Trans. 1999, 63–66. [Google Scholar]
- Fischer, T.; Sethi, A.; Welton, T.; Woolf, J. Diels-Alder reactions in room-temperature ionic liquids. Tetrahedron Lett. 1999, 40, 793–796. [Google Scholar] [CrossRef]
- Earle, M.J.; McCormac, P.B.; Seddon, K.R. Regioselective alkylation in ionic liquids. Chem. Commun. 1998, 2245–2246. [Google Scholar]
- Monteiro, A.L.; Zinn, F.K.; de Souza, R.F.; Dupont, J. Asymmetric hydrogenation of 2-arylacrylic acids catalyzed by immobilized Ru-BINAP complex in 1-n-butyl-3-methylimidazolium tetra-fluoroborate molten salt. Tetrahedron Asymmetry 1997, 8, 177–179. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Bohm, V.P. Heck reaction catalyzed by phospha-palladacycles in non-aqueous ionic liquids. J. Organomet. Chem. 1999, 572, 141–145. [Google Scholar] [CrossRef]
- Dupont, J.; Silva, S.M.; de Souza, R.F. Mobile phase effects in Rh/sulfonated phosphine/molten salts catalysed the biphasic hydroformylation of heavy olefins. Catal. Lett. 2001, 77, 131–133. [Google Scholar] [CrossRef]
- Bernardo-Gusmão, K.; Queiroz, L.F.T.; de Souza, R.F.; Leca, F.; Loup, C.; Réau, R. Biphasic oligomerization of ethylene with nickel–1,2-diiminophosphorane complexes immobilized in 1-n-butyl-3-methylimidazolium organochloroaluminate. J. Catal. 2003, 219, 59–62. [Google Scholar] [CrossRef]
- Crosthwaite, J.M.; Farmer, V.A.; Hallett, J.P; Welton, T. Epoxidation of alkenes by Oxone™ using 2-alkyl-3,4-dihydroisoquinolinium salts as catalysts in ionic liquids. J. Mol. Catal. A Chem. 2008, 279, 148–152. [Google Scholar] [CrossRef]
- Freedman, M.L. The tungstic acids. J. Am. Chem. Soc. 1959, 81, 3834–3839. [Google Scholar] [CrossRef]
- Maiti, S.K.; Banerjee, S.; Mukherjee, A.K.; Abdul Malik, K.M.; Bhattacharyya, R. Oxoperoxo molybdenum(VI) and tungsten(VI) and oxodiperoxo molybdate(VI) and tungstate(VI) complexes with 8-quinolinol: Synthesis, structure and catalytic activity. New J. Chem. 2005, 29, 554–563. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Biswas, S.; Guha, S.; Mukherjee, A.K.; Bhattacharyya, R. Novel oxo-peroxo molybdenum(VI) complexes incorporating 8-quinolinol: Synthesis, structure and catalytic uses in the environmentally benign and cost-effective oxidation method of methyl benzenes: Ar(CH3)n (n = 1, 2). Chem. Comm. 1999, 1627–1628. [Google Scholar]
- Yao, H.; Richardson, D.E. Epoxidation of alkenes with bicarbonate-activated hydrogen peroxide. J. Am.Chem. Soc. 2000, 122, 3220–3221. [Google Scholar] [CrossRef]
- Lane, B.S.; Vogt, M.; De Rose, V.J.; Burgess, K. Manganese-catalyzed epoxidations of alkenes in bicarbonate solutions. J. Am. Chem. Soc. 2002, 124, 11946–11954. [Google Scholar] [CrossRef]
- Chinese Standard GB 1677-2008. In Determination of the epoxy value of plasticizers; General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China & Standardization Administration of the People's Republic of China: Beijing, China, 2008.
- Chinese Standard GB/T 1668-2008. In Plasticizers-Determination of acid value and acidity; General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China & Standardization Administration of the People's Republic of China: Beijing, China, 2008.
- Sample Availability: Samples of the compounds MoO3·2H2O and [MoO(O2)2·2QOH] (QOH = 8-quinilinol) are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cai, S.-F.; Wang, L.-S.; Fan, C.-L. Catalytic Epoxidation of a Technical Mixture of Methyl Oleate and Methyl Linoleate in Ionic Liquids Using MoO(O2)2•2QOH (QOH = 8-quinilinol) as Catalyst and NaHCO3 as co-Catalyst. Molecules 2009, 14, 2935-2946. https://doi.org/10.3390/molecules14082935
Cai S-F, Wang L-S, Fan C-L. Catalytic Epoxidation of a Technical Mixture of Methyl Oleate and Methyl Linoleate in Ionic Liquids Using MoO(O2)2•2QOH (QOH = 8-quinilinol) as Catalyst and NaHCO3 as co-Catalyst. Molecules. 2009; 14(8):2935-2946. https://doi.org/10.3390/molecules14082935
Chicago/Turabian StyleCai, Shuang-Fei, Li-Sheng Wang, and Chuan-Lei Fan. 2009. "Catalytic Epoxidation of a Technical Mixture of Methyl Oleate and Methyl Linoleate in Ionic Liquids Using MoO(O2)2•2QOH (QOH = 8-quinilinol) as Catalyst and NaHCO3 as co-Catalyst" Molecules 14, no. 8: 2935-2946. https://doi.org/10.3390/molecules14082935




