Intramolecular Transformations of 3-Cyanoamino- and 3-Cyanoimino-1,2-diferrocenylcyclopropenes
Abstract
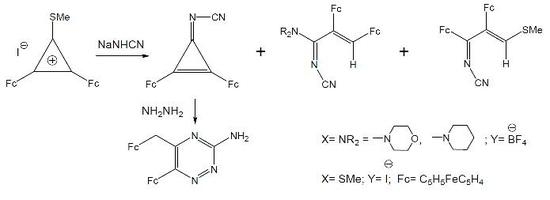
:Introduction

Results and Discussion


| Selected bond lengths (Å) | Selected bond angles (o) | ||
|---|---|---|---|
| 7a | |||
| C(24)-N(2) | 1.151(4) | N(2)-C(24)-N(1) | 172.6(3) |
| C(24)-N(1) | 1.329(4) | C(23)-N(1)-C(24) | 119.3(2) |
| C(23)-N(1) | 1.314(3) | N(1)-C(23)-N(3) | 117.5(2) |
| C(23)-C(22) | 1.495(3) | N(1)-C(23)-C(22) | 123.2(2) |
| C(22)-C(21) | 1.334(3) | C(23)-C(22)-C(21) | 118.8(2) |
| C(23)-N(3) | 1.332(3) | C(22)-C(23)-N(3) | 119.3(2) |
| N(3)-C(25) | 1.454(4) | C(21)-C(22)-C(11) | 123.0(2) |
| C(1)-C(21) | 1.462(3) | C(23)-N(3)-C(27) | 121.4(3) |
| 10 | |||
| N(1)-C(25) | 1.338(6) | C(21)-N(1)-C(25) | 120.1(4) |
| N(2)-C(25) | 1.145(6) | N(2)-C(25)-N(1) | 172.1(5) |
| C(21)-N(1) | 1.303(5) | N(1)-C(21)-C(22) | 122.7(3) |
| C(21)-C(22) | 1.499(5) | C(21)-C(22)-C(23) | 115.8(3) |
| C(22)-C(23) | 1.350(5) | C(22)-C(23)-S(1) | 126.9(3) |
| C(23)-S(1) | 1.739(4) | C(23)-S(1)-C(24) | 100.6(2) |
| S(1)-C(24) | 1.789(5) | N(1)-C(21)-C(1) | 118.5(4) |
| C(22)-C(11) | 1.459(5) | C(1)-C(21)-C(22) | 118.8(3) |
| C(1)-C(21) | 1.442(5) | C(11)-C(22)-C(23) | 127.8(3) |









Experimental
General
Synthesis of diferrocenylcyclopropenylium salts (5a, 5b, and 2)
Reaction of dialkylamino(diferrocenyl)cyclopropenylium tetrafluoroborates with sodium hydrogencyanamide
Reaction of 2,3-diferrocenyl-1-methylthiocyclopropenylium iodide (2) with sodium hydrogencyanamide
Reaction of 3-cyanimino-1,2-diferrocenylcyclopropene (8) with hydrazine
Transformation of 3-dialkylamino-, 3-methylthio-3-cyanamino-1,2-diferrocenylcyclopropenes 6a,b and 11a into 3-cyanimino-1,2-diferrocenylcyclopropene (8)
Transformation of 3-cyanamino-2,3-diferrocenyl-1-methylthiocyclopropene (11b) into Z-3-cyanoimino-2,3-diferrocenyl-1-methylthioprop-1-ene (10)
Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds 5a,b, 7a,b and 8 are available from the authors.
References and Notes
- Corey, E.J.; Jautelat, M. Construction of Ring Systems Containing the gem-Dimethylcyclopropane Unit Using Diphenylsulfonium Isopropylide. J. Am. Chem. Soc. 1967, 89, 3912–3914. [Google Scholar]
- Burce, S.D.; Grieco, P.A. Intramolecular Reactions of Diazocarbonyl Compounds. Org. React. 1979, 26, 361–475. [Google Scholar]
- Gant, T.G.; Noe, M.C.; Corey, E.J. The First Enantioselective Synthesis of the Chemotactic Factor Sirenin by an Intramolecular [2+1] Cyclization Using a New Chiral Catalyst. Tetrahedron Lett. 1995, 36, 8745–8748. [Google Scholar] [CrossRef]
- Hudlicky, T.; Koszyk, F.F.; Kutchan, T.M.; Sheth, J.P. Cyclopentene Annulation via Intramolecular Addition of Diazo Ketones to 1,3-Dienes. Applications to the Synthesis of Cyclopentanoid Terpenes. J. Org. Chem. 1980, 45, 5020–5027. [Google Scholar]
- Flynn, B.L.; de Meijere, A. Unprecedented Regio- and Stereoselective Conversi-on of 1-Cyclopropyl-3-ethoxycyclopentadienes to 3-(E)-Alkylidenecyclopentenes. J. Org. Chem. 1999, 64, 400–404. [Google Scholar] [CrossRef]
- Komatsu, K.; Kitagawa, T. Cyclopropenylium Cations, Cyclopropenones, and Heteroanaloges - Recent Advances. Chem. Rev. 2003, 103, 1371–1427. [Google Scholar]
- Potts, K.T.; Baum, J.S. The Chemistry of Cyclopropenones. Chem. Rev. 1974, 74, 189–213. [Google Scholar]
- Billups, W. The Chemistry of the Cyclopropyl Group; Patai, S., Rappoport, A., Eds.; Wiley Interscience: New York, NY, USA, 1987. [Google Scholar]
- Postnov, V.N.; Klimova, E.I.; Meleshonkova, N.N.; Bolesov, I.G. On the opening of cyclopropanes of ferrocenyl compounds. Dokl. Akad. Nauk 1994, 339, 496–498. [Google Scholar]
- Nesmeyanov, A.N.; Klimova, E.I.; Struchkov, Yu. T.; Andrianov, V.G.; Postnov, V.N.; Sazonova, V.A. Cyclopropanes with a ferrocenyl group. J. Organomet. Chem. 1979, 178, 343–348. [Google Scholar]
- Agranat, I.; Ahron-Shalom, E.; Kriegerm, R.L.; Krug, W.O. Stabilization of the cyclopropenum and cyclopropenone ring systems by ferrocene. Tetrahedron 1979, 35, 733–740. [Google Scholar] [CrossRef]
- Klimova, E.I.; Klimova-Berestneva, T.; Ruiz-Ramirez, L.; Martinez-Garcia, M.; Alvarez-Toledano, C.; Espinosa, P.G.; Toscano, R.A. Structure of Z- and E-2-bromo-1-ferrocenyl-1-phenylcyclopropanes and 3-ferrocenyl-3-phenylcyclopropene and their three-membered ring opening reactions. J. Organomet. Chem. 1997, 545-546, 191–199. [Google Scholar]
- Klimova, T.; Klimova, E.I.; Alvarez-Toledano, C.; Toscano, R.A.; Martinez-Garcia, M. 1-Ferrocenylcyclopropene and 1-ferrocenylcyclopropyl cation. J. Organomet. Chem. 2003, 665, 23–28. [Google Scholar]
- Klimova, E.I.; Martínez-García, M.; Klimova, T.; Mendez-Stivalet, J.M.; Hernandez-Ortega, S.; Ruiz-Ramirez, L. 3,3-Diferrocenylcyclopropene. J. Organomet. Chem. 2002, 659, 56–63. [Google Scholar]
- Abram, T.S.; Watts, W.I. Stable Carbocations. Part VIII. Fragmentation Reactions of Ferrocenylalkylium Ions. J. Chem. Soc. Perkin Trans. I 1975, 113–116. [Google Scholar]
- Klimova-Berestneva, T.; Klimova, E.I.; Mendez-Stivalet, J.M.; Hernandez-Ortega, S.; Martinez-Garcia, M. Diferrocenyl(methylthio)cyclopropenylium iodide in the synthesis of 2,3-diferrocenyl-1-methylthio-1,3-dienes and -1,3,5-trienes. Eur. J. Org. Chem. 2005, 4406–4413. [Google Scholar]
- Klimova, E.I.; Klimova-Berestneva, T.; Hernandez-Ortega, S.; Ortiz-Frade, L.; Backi-nowsky, L.V.; Martínez-García, M. Synthesis of 1,2-diferrocenyl-3-(diacylme-thylidene)cyclopropenes and 1,1-diacyl-2,3-diferrocenyl-4-methylsulfanylbuta-1,3-dienes, their structures and electrochemical properties. J. Organomet. Chem. 2008, 693, 1215–1224. [Google Scholar]
- Klimova, E.I.; Martínez-García, M.; Klimova-Berestneva, T.; Alvarez-Toledano, C.; Toscano, R.A.; Backinowsky, L.V. Functional Group Migration in Reactions of Diferrocenyl(methylthio)cyclopropenylium Iodide with CH-acids. Eur. J. Org. Chem. 2006, 4755–4760. [Google Scholar]
- Klimova-Berestneva, T.; Klimova, E.I.; Flores -Alamo, M.; Backinowsky, L.V.; Martìnez-Garcìa, M. Formation of 4,5-diferrocenyl-6-methylthio-6H-1,2-oxazine N-oxides and Migration of a Nitro Group in Reactions of 2,3-Diferrocenyl-1-methylthiocyclopropenylium iodide with Nitroalkanes. Synthesis 2006, 3706–3710. [Google Scholar]
- Klimova, E.I.; Klimova, T.; Ruiz-Ramirez, L.; Cinquantini, A.; Corsini, M.; Zanello, P.; Hernandez-Ortega, S.; Martinez-Garcia, M. 2,3-Diferrocenylcyclopropenone: Synthesis, structure and some chemical and electrochemical properties. Eur. J. Org. Chem. 2003, 4265–4272. [Google Scholar]
- Klimova, E.I.; Klimova-Berestneva, T.; Hernández-Ortega, S.; Méndez-Iturbide, D.; García-Marquez, A.; Martínez-García, M. Diferrocenylcyclopropenyl cations. Synthesis, structures, and some chemical properties. J. Organomet. Chem. 2005, 690, 3332–3339. [Google Scholar]
- CCDC 72930 (for 7a) and 729319 (for 10) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge at www.ccdc.cam.ac.uk/const/retrieving.html [or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge DB2 1EZ, UK; Fax: (internat.) +44-1223/336-033; E-mail: [email protected]]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed; Cornell University Press: Ithaca, NY, USA, 1960; pp. 228–282. [Google Scholar]
- Ku, A.T.; Sundaraligam, M. X-Ray Studies on Cyclopropenyl Cations. II. Crystal and Molecular Structure of 1,2,3-Trisdimethylaminocyclopropenium Perchlorate. J. Am. Chem. Soc. 1972, 94, 1688–1692. [Google Scholar] [CrossRef]
- Postnov, V.N.; Klimova, E.I.; Pushin, A.N.; Meleshonkova, N.N. The interaction of the 1,3-bis(p-methoxyphenyl)allylic cation with ferrocenyl-1,3-butadienes. Metalloorg. Khim. 1992, 5, 564–569. [Google Scholar]
- Hückel, E. Quantum theoretical contributions to the problem of aromatic and non-saturated compounds. Z. Physik. 1932, 76, 628–648. [Google Scholar] [CrossRef]
- Wiberg, K.B. Antiaromaticity in Monocyclic Conjugated Carbon Rings. Chem. Rev. 2001, 101, 1317–1331. [Google Scholar]
- Robin, M.B.; Day, P. Mixed valence chemistry. A survey and classification. Adv. Inorg. Chem. Radiochem. 1967, 10, 247–422. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97, Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1994. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Klimova, E.I.; Klimova, T.; Flores-Alamo, M.; Backinowsky, L.V.; Garcia, M.M. Intramolecular Transformations of 3-Cyanoamino- and 3-Cyanoimino-1,2-diferrocenylcyclopropenes. Molecules 2009, 14, 3161-3175. https://doi.org/10.3390/molecules14093161
Klimova EI, Klimova T, Flores-Alamo M, Backinowsky LV, Garcia MM. Intramolecular Transformations of 3-Cyanoamino- and 3-Cyanoimino-1,2-diferrocenylcyclopropenes. Molecules. 2009; 14(9):3161-3175. https://doi.org/10.3390/molecules14093161
Chicago/Turabian StyleKlimova, Elena Ivanovna, Tatiana Klimova, Marcos Flores-Alamo, Leon Vladimirovich Backinowsky, and Marcos Martinez Garcia. 2009. "Intramolecular Transformations of 3-Cyanoamino- and 3-Cyanoimino-1,2-diferrocenylcyclopropenes" Molecules 14, no. 9: 3161-3175. https://doi.org/10.3390/molecules14093161