Synergistic Chondroprotective Effect of α-Tocopherol, Ascorbic Acid, and Selenium as well as Glucosamine and Chondroitin on Oxidant Induced Cell Death and Inhibition of Matrix Metalloproteinase-3—Studies in Cultured Chondrocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Potential cytotoxic effects of the test substances and IL-1β

2.2. Effects of α‑tocopherol, ascorbic acid and selenium on tert‑butyl hydroperoxide induced cell death

| t-BHP | 100 µmol/L | 200 µmol/L | 500 µmol/L | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SEM | M | SEM | M | SEM | |||||
| Control | 49.7 | 4.0 | 37.6 | 3.2 | 31.4 | 1.2 | ||||
| α‑Tocopherol (α‑toc)µmol/L | ||||||||||
| 0.1 | 65.4 | 5.4 | 49.3 | 4.6 | 36.4a | 1.6 | ||||
| 0.5 | 90.7c | 3.8 | 79.1c | 7.9 | 64.8c | 3.3 | ||||
| 2.5 | 92.8c | 3.2 | 89.7c | 1.4 | 83.9c | 7.5 | ||||
| Ascorbic acid (AA) µmol/L | ||||||||||
| 10 | 60.8 | 3.6 | 43.1 | 2.2 | 32.4 | 1.0 | ||||
| 25 | 78.2c | 2.6 | 59.6b | 4.1 | 36.1 | 0.7 | ||||
| 50 | 73.0b | 4.5 | 65.9c | 4.6 | 37.5a | 1.8 | ||||
| Selenium (Se) nmol/L | ||||||||||
| 1 | 53.7 | 4.3 | 39.4 | 3.1 | 29.0 | 1.2 | ||||
| 10 | 77.9b | 5.5 | 55.4b | 4.6 | 33.2 | 1.2 | ||||
| 50 | 91.6c | 4.1 | 83.0c | 1.7 | 47.8b | 4.2 | ||||
| Combinations | ||||||||||
| I (0.1 µmol/L α‑toc, 10 µmol/L AA, 1 nmol/L Se) | 87.0c | 3.6 | 74.2b | 6.1 | 42.6a | 3.1 | ||||
| II (0.1 µmol/L α‑toc, 25 µmol/L AA, 10 nmol/L Se) | 98.1c | 3.2 | 89.6c | 1.6 | 69.0c | 4.6 | ||||
| III (0.1 µmol/L α‑toc, 25 µmol/L AA, 25 nmol/L Se) | 100.6c | 1.2 | 95.2c | 2.4 | 82.7c | 1.0 | ||||
| IV (0.5 µmol/L α‑toc, 25 µmol/L AA, 10 nmol/L Se) | 104.8c | 1.6 | 97.8c | 2.8 | 93.6c | 3.9 | ||||
| V (0.1 µmol/L α‑toc, 50 µmol/L AA, 50 nmol/L Se) | 99.3c | 2.7 | 96.5c | 2.8 | 94.4c | 2.3 | ||||
| VI (0.5 µmol/L α‑toc, 50 µmol/L AA, 50 nmol/L Se) | 101.7c | 4.1 | 101.5c | 1.6 | 94.8c | 2.0 | ||||
| VII (2.5 µmol/L α‑toc, 50 µmol/L AA, 50 nmol/L Se) | 103.0c | 1.2 | 98.9c | 2.9 | 98.0c | 4.5 | ||||
2.3. mRNA levels and secretion of matrix metalloproteinase-3
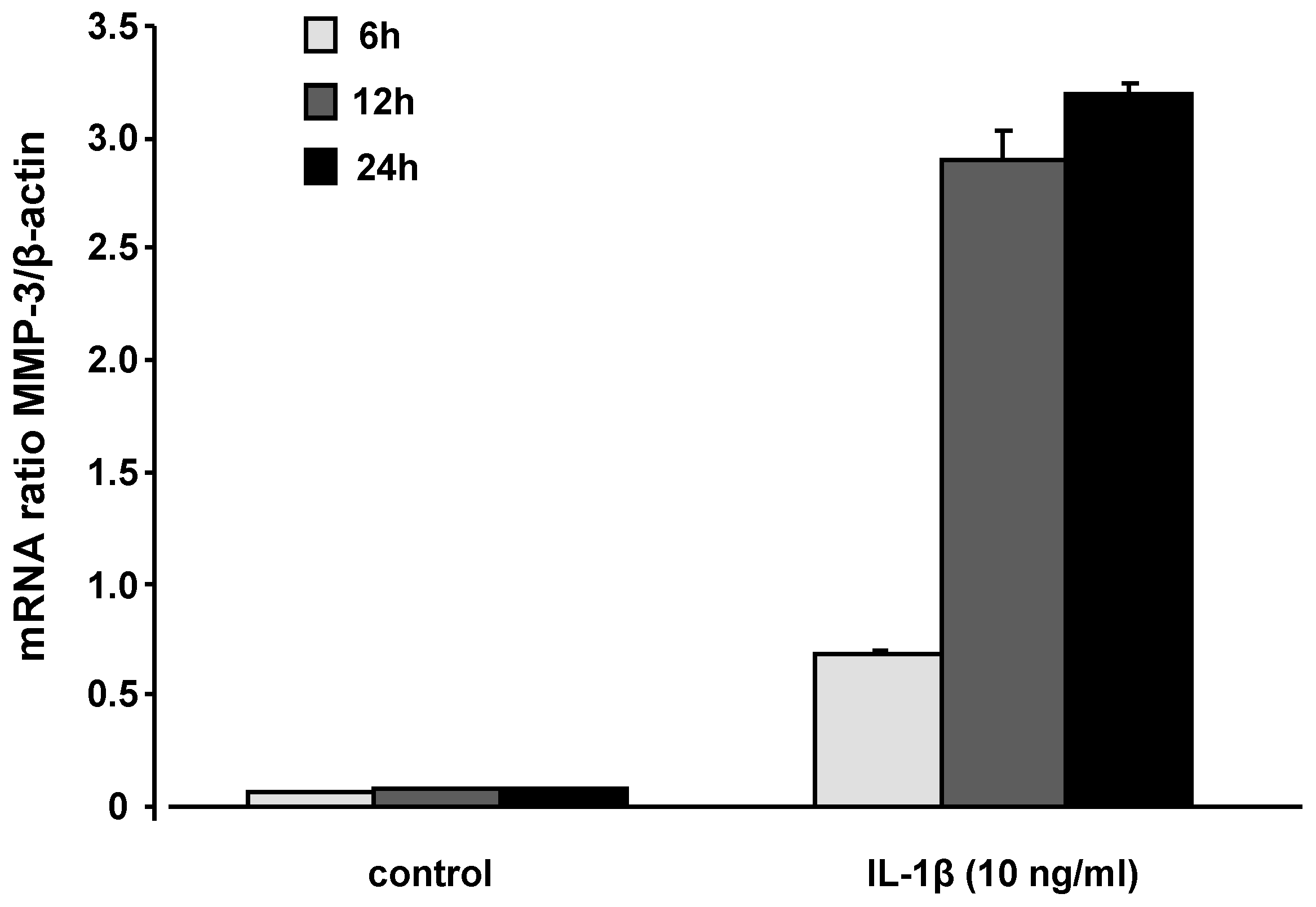


3. Experimental
3.1. Cell culture
3.2. Cytotoxicity studies
3.3. Matrix metalloproteinase-3 and tumor necrosis factor alpha gene expression and secretion
RNA isolation and real time PCR measurements
| Gene | Sequence (5' - 3') | Annealing temperature |
|---|---|---|
| b-actin | F: GGA TGC AGA AGG AGA TCA CTG | 55°C |
| R: CGA TCC ACA CGC AGT ACT TG | ||
| MMP3 | F: TTT TGG CCA TCT CTT CCT TCA | 59°C |
| R: TGT GGA TGC CTC TTG GGT ATC | ||
| TNF-α | F: CCC CAG GGA CCT CTC TCT A | 60°C |
| R: GGT TTG CTA CAA CAT GGG CTA CA |
3.4. MMP3 and TNFα quantification by ELISA
3.5. Western blot analysis for p65
3.5. Statistical analysis
4. Conclusions
- Sample Availability: Contact the authors.
References
- Pattoli, M.A.; MacMaster, J.F.; Gregor, K.R.; Burke, J.R. Collagen and aggrecan degradation is blocked in interleukin-1-treated cartilage explants by an inhibitor of IkappaB kinase through suppression of metalloproteinase expression. J. Pharmacol. Exp. Ther. 2005, 315, 382–388. [Google Scholar] [CrossRef]
- Choi, E.J.; Bae, S.C.; Yu, R.; Youn, J.; Sung, M.K. Dietary vitamin E and quercetin modulate inflammatory responses of collagen-induced arthritis in mice. J. Med. Food. 2009, 12, 770–775. [Google Scholar] [CrossRef]
- Kurz, B.; Jost, B.; Schünke, M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarth. Cartilage 2002, 10, 119–126. [Google Scholar] [CrossRef]
- van Vugt, R.M.; Rijken, P.J.; Rietveld, A.G.; van Vugt, A.C.; Dijkmans, B.A. Antioxidant intervention in rheumatoid arthritis: Results of an open pilot study. Clin. Rheumatol. 2008, 27, 771–775. [Google Scholar]
- Bae, S.C.; Kim, S.J.; Sung, M.K. Inadequate antioxidant nutrient intake and altered plasma antioxidant status of rheumatoid arthritis patients. J. Am. Coll. Nutr. 2003, 22, 311–315. [Google Scholar]
- Wang, Y.; Hodge, A.M.; Wluka, A.E.; English, D.R.; Giles, G.G.; O'Sullivan, R.; Forbes, A.; Cicuttini, F.M. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: A cross-sectional study. Arthritis Res. Ther. 2007, 9, R66. [Google Scholar] [CrossRef]
- Dodge, G.R.; Jimenez, S.A. Glucosamine sulfate modulates the levels of aggrecan and matrix metalloproteinase-3 synthesized by cultured human osteoarthritis articular chondrocytes. Osteoarth. Cartilage 2003, 11, 424–432. [Google Scholar] [CrossRef]
- Legendre, F.; Baugé, C.; Roche, R.; Saurel, A.S.; Pujol, J.P. Chondroitin sulfate modulation of matrix and inflammatory gene expression in IL-1beta-stimulated chondrocytes--study in hypoxic alginate bead cultures. Osteoarth. Cartilage 2008, 16, 105–114. [Google Scholar] [CrossRef]
- Shikhman, A.R.; Kuhn, K.; Alaaeddine, N.; Lotz, M. N-acetylglucosamine prevents IL-1 beta-mediated activation of human chondrocytes. J. Immunol. 2001, 166, 5155–5160. [Google Scholar]
- Hitchon, C.A.; El-Gabalawy, H.S. Oxidation in rheumatoid arthritis. Arthritis Res. Ther. 2004, 6, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J. Nutr. 2001, 131, 369–373. [Google Scholar]
- Rimbach, G.; Minihane, A.M.; Majewicz, J.; Fischer, A.; Pallauf, J.; Virgli, F.; Weinberg, P.D. Regulation of cell signalling by vitamin E. Proc. Nutr. Soc. 2002, 61, 415–425. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Roveri, A. Phospholipid hydroperoxide glutathione peroxidase (PHGPx): More than an antioxidant enzyme? Biomed. Environ. Sci. 1997, 10, 327–332. [Google Scholar]
- Fischer, A.; Pallauf, J.; Gohil, K.; Weber, S.U.; Packer, L.; Rimbach, G. Effect of selenium and vitamin E deficiency on differential gene expression in rat liver. Biochem. Biophys. Res. Commun. 2001, 285, 470–475. [Google Scholar]
- Allen, N.E.; Appleby, P.N.; Roddam, A.W.; Tjønneland, A.; Johnsen, N.F.; Overvad, K.; Boeing, H.; Weikert, S.; Kaaks, R.; Linseisen, J.; Trichopoulou, A.; Misirli, G.; Trichopoulos, D.; Sacerdote, C.; Grioni, S.; Palli, D.; Tumino, R.; Bueno-de-Mesquita, H.B.; Kiemeney, L.A.; Barricarte, A.; Larrañaga, N.; Sánchez, M.J.; Agudo, A.; Tormo, M.J.; Rodriguez, L.; Stattin, P.; Hallmans, G.; Bingham, S.; Khaw, K.T.; Slimani, N.; Rinaldi, S.; Boffetta, P.; Riboli, E.; Key, T.J. Plasma selenium concentration and prostate cancer risk: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Clin. Nutr. 2008, 88, 1567–1575. [Google Scholar]
- Bader, N.; Bosy-Westphal, A.; Koch, A.; Rimbach, G.; Weimann, A.; Poulsen, H.E.; Müller, M.J. Effect of hyperbaric oxygen and vitamin C and E supplementation on biomarkers of oxidative stress in healthy men. Br. J. Nutr. 2007, 98, 826–833. [Google Scholar]
- Behrens, W.A.; Madere, R. Alpha- and gamma tocopherol concentrations in human serum. J. Am. Coll. Nutr. 1986, 5, 91–96. [Google Scholar]
- Majewicz, J.; Rimbach, G.; Proteggente, A.R.; Lodge, J.K.; Kraemer, K.; Minihane, A.M. Dietary vitamin C down-regulates inflammatory gene expression in apoE4 smokers. Biochem. Biophys. Res. Commun. 2005, 338, 951–955. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Rota, C.; Majewicz, J.; Rimbach, G.; Minihane, A.M.; Kramer, K.; Lodge, J.K. Cigarette smokers differ in their handling of natural (RRR) and synthetic (all rac) alpha-tocopherol: A biokinetik study in apoE4 male subjects. Free Radic Biol. Med. 2006, 40, 2080–2091. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef]
- Persiani, S.; Roda, E.; Rovati, L.C.; Locatelli, M.; Giacovelli, G.; Roda, A. Glucosamine oral bioavailability and plasma pharmacokinetics after increasing doses of crystalline glucosamine sulfate in man. Osteoarth. Cartilage 2005, 13, 1041–1049. [Google Scholar] [CrossRef]
- Volpi, N. Oral bioavailability of chondroitin sulfate (Condrosulf) and its constituents in healthy male volunteers. Osteoarth. Cartilage 2002, 10, 768–777. [Google Scholar] [CrossRef]
- Daheshia, M.; Yao, J.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar] [CrossRef]
- Gebauer, M.; Saas, J.; Sohler, F.; Haag, J.; Söder, S.; Pieper, M.; Bartnik, E.; Beninga, J.; Zimmer, R.; Aigner, T. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1beta. Osteoarth. Cartilage 2005, 13, 697–708. [Google Scholar] [CrossRef]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Aspects Med. 2008, 29, 290–308. [Google Scholar] [CrossRef]
- Sandy, J.D.; Thompson, V.; Verscharen, C.; Gamett, D. Chondrocyte-mediated catabolism of aggrecan: Evidence for a glycosylphosphatidylinositol-linked protein in the aggrecanase response to interleukin-1 or retinoic acid. Arch. Biochem. Biophys. 1999, 367, 258–264. [Google Scholar] [CrossRef]
- Rauchman, M.I.; Wasserman, J.C.; Cohen, D.M.; Perkins, D.L.; Hebert, S.C.; Milford, E.; Gullans, S.R. Expression of GLUT-2 cDNA in human B lymphocytes: Analysis of glucose transport using flow cytometry. Biochim. Biophys. Acta 1992, 1111, 231–238. [Google Scholar] [CrossRef]
- Gouze, J.N.; Bianchi, A.; Bécuwe, P.; Dauça, M.; Netter, P.; Magdalou, J.; Terlain, B.; Bordji, K. Glucosamine modulates IL-1-induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF-kappa B pathway. FEBS Lett. 2002, 510, 166–170. [Google Scholar] [CrossRef]
- Bond, M.; Baker, A.H.; Newby, A.C. Nuclear factor kappaB activity is essential for matrix metalloproteinase-1 and -3 upregulation in rabbit dermal fibroblasts. Biochem. Biophys. Res. Commun. 1999, 264, 561–567. [Google Scholar] [CrossRef]
- Iovu, M.; Dumais, G.; du Souich, P. Anti-inflammatory activity of chondroitin sulfate. Osteoarth. Cartilage 2008, 16, 14–18. [Google Scholar]
- Klatt, A.R.; Klinger, G.; Paul-Klausch, B.; Kühn, G.; Renno, J.H.; Wagener, R.; Paulsson, M.; Schmidt, J.; Malchau, G.; Wielckens, K. Matrilin-3 activates the expression of osteoarthritis-associated genes in primary human chondrocytes. FEBS Lett. 2009, 583, 3611–3617. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Valacchi, G.; Rimbach, G.; Saliou, C.; Weber, S.U.; Packer, L. Effect of benzoyl peroxide on antioxidant status, NF-kappaB activity and interleukin-1alpha gene expression in human keratinocytes. Toxicology 2001, 165, 225–234. [Google Scholar] [CrossRef]
- Wagner, A.E.; Ernst, I.; Iori, R.; Desel, C.; Rimbach, G. Sulforaphane but not ascorbigen, indole-3-carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture. Exp. Dermatol. 2009. [Epub ahead of print]. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Graeser, A.-C.; Giller, K.; Wiegand, H.; Barella, L.; Boesch Saadatmandi, C.; Rimbach, G. Synergistic Chondroprotective Effect of α-Tocopherol, Ascorbic Acid, and Selenium as well as Glucosamine and Chondroitin on Oxidant Induced Cell Death and Inhibition of Matrix Metalloproteinase-3—Studies in Cultured Chondrocytes. Molecules 2010, 15, 27-39. https://doi.org/10.3390/molecules15010027
Graeser A-C, Giller K, Wiegand H, Barella L, Boesch Saadatmandi C, Rimbach G. Synergistic Chondroprotective Effect of α-Tocopherol, Ascorbic Acid, and Selenium as well as Glucosamine and Chondroitin on Oxidant Induced Cell Death and Inhibition of Matrix Metalloproteinase-3—Studies in Cultured Chondrocytes. Molecules. 2010; 15(1):27-39. https://doi.org/10.3390/molecules15010027
Chicago/Turabian StyleGraeser, Anne-Christi, Katri Giller, Heike Wiegand, Luca Barella, Christine Boesch Saadatmandi, and Gerald Rimbach. 2010. "Synergistic Chondroprotective Effect of α-Tocopherol, Ascorbic Acid, and Selenium as well as Glucosamine and Chondroitin on Oxidant Induced Cell Death and Inhibition of Matrix Metalloproteinase-3—Studies in Cultured Chondrocytes" Molecules 15, no. 1: 27-39. https://doi.org/10.3390/molecules15010027





