Prenylated Xanthones from the Bark of Garcinia xanthochymus and Their 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activities
Abstract
:1. Introduction
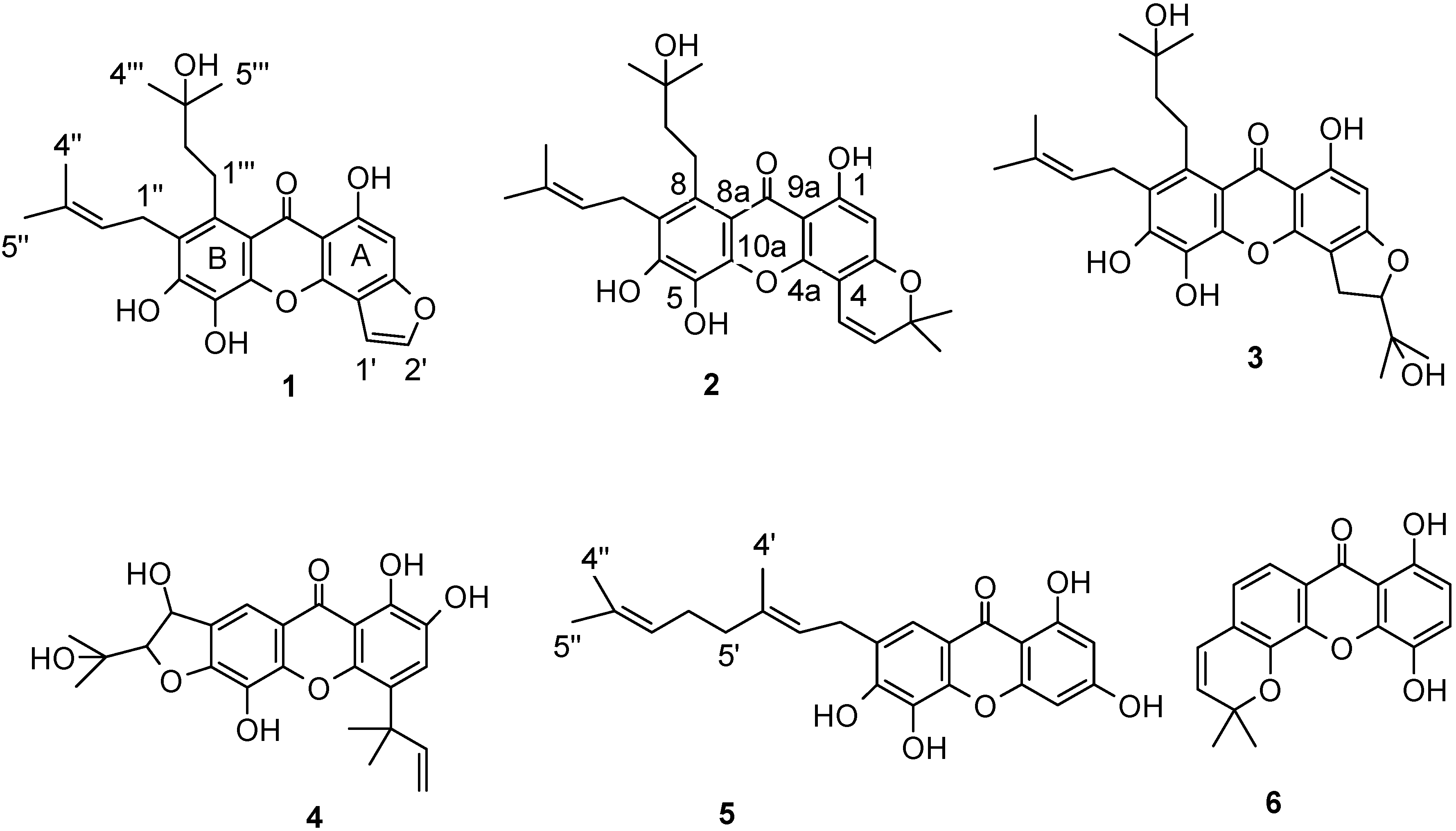
2. Results and Discussion
2.1. Structural elucidations of xanthones

| Position | 1 | 2 | 3 | 6 |
|---|---|---|---|---|
| 1-OH | 13.61 s | 14.03 s | 12.02 s | |
| 5-OH | 8.90 s | 9.43 s | ||
| 6-OH | 9.12 s | 9.82 s | ||
| 2 | 6.85 s | 6.14 s | 6.19 s | 6.64 d (8.9) |
| 3 | 7.32 d (8.9) | |||
| 7 | 7.18 d (8.1) | |||
| 8 | 7.72 d (8.1) | |||
| 1′ | 7.39 br s | 7.11 d (9.3) | 3.27 m | 6.59 d (9.8) |
| 2′ | 7.80 br s | 5.72 d (9.3) | 4.77 m | 6.02 d (9.8) |
| 4′ | 1.42 s | 1.17 s | 1.54 s | |
| 5′ | 1.42 s | 1.17 s | 1.54 s | |
| 1′′ | 3.56 d (5.6) | 3.35 d (6.0) | 3.39 d (5.4) | |
| 2′′ | 5.14 br s | 5.15 br s | 5.02 br s | |
| 4′′ | 1.83 s | 1.84 s | 1.77 s | |
| 5′′ | 1.68 s | 1.70 s | 1.65 s | |
| 1′′′ | 3.45 m | 3.44 m | 3.34 m | |
| 2′′′ | 1.76 m | 1.74 m | 1.55 m | |
| 4′′′ | 1.32 s | 1.32 s | 1.20 s | |
| 5′′′ | 1.32 s | 1.32 s | 1.20 s |
| Position | 1 | 2 | 3 | 6 |
|---|---|---|---|---|
| 1 | 160.9 (qC) | 163.8 (qC) | 163.7 (qC) | 154.2 (qC) |
| 2 | 94.1 (CH) | 99.0 (CH) | 92.4 (CH) | 109.6 (CH) |
| 3 | 160.6 (qC) | 160.3 (qC) | 166.7 (qC) | 124.5 (CH) |
| 4 | 108.5 (qC) | 104.0 (qC) | 102.5 (qC) | 138.2 (qC) |
| 4a | 146.1 (qC) | 150.9 (qC) | 150.4 (qC) | 144.6 (qC) |
| 5 | 130.3 (qC) | 130.0 (qC) | 129.7 (qC) | 141.7 (qC) |
| 10a | 149.4 (qC) | 146.8 (qC) | 145.8 (qC) | 146.1 (qC) |
| 6 | 150.5 (qC) | 151.3 (qC) | 150.6 (qC) | 127.6 (qC) |
| 7 | 126.1 (qC) | 125.6 (qC) | 124.8 (qC) | 122.3 (CH) |
| 8 | 136.4 (qC) | 136.7 (qC) | 134.9 (qC) | 117.3 (CH) |
| 8a | 112.0 (qC) | 111.6 (qC) | 110.1 (qC) | 121.5 (qC) |
| 9 | 183.8 (qC) | 183.1 (qC) | 181.9 (qC) | 182.4 (qC) |
| 9a | 105.8 (qC) | 101.1 (qC) | 103.3 (qC) | 109.4 (qC) |
| 1′ | 104.9 (CH) | 115.9 (CH) | 26.7 (CH2) | 122.1 (CH) |
| 2′ | 144.8 (CH) | 127.2 (CH) | 91.6 (CH) | 134.9 (CH) |
| 3′ | 78.3 (qC) | 70.0 (qC) | 78.6 (qC) | |
| 4′ | 28.0 (CH3) | 26.0 (CH3) | 27.6 (CH3) | |
| 5′ | 28.0 (CH3) | 24.9 (CH3) | 17.6 (CH3) | |
| 1′′ | 25.2 (CH2) | 25.1 (CH2) | 24.1 (CH2) | |
| 2′′ | 123.7 (CH) | 123.7 (CH) | 123.3 (CH) | |
| 3′′ | 131.5 (qC) | 131.4 (qC) | 130.6 (qC) | |
| 4′′ | 18.0 (CH3) | 17.9 (CH3) | 18.0 (CH3) | |
| 5′′ | 25.6 (CH3) | 25.6 (CH3) | 25.6 (CH3) | |
| 1′′′ | 24.8 (CH2) | 24.8 (CH2) | 24.5 (CH2) | |
| 2′′′ | 45.3 (CH2) | 45.3 (CH2) | 44.9 (CH2) | |
| 3′′′ | 70.0 (qC) | 69.9 (qC) | 69.0 (qC) | |
| 4′′′ | 28.9 (CH3) | 28.9 (CH3) | 29.1 (CH3) | |
| 5′′′ | 28.9 (CH3) | 28.9 (CH3) | 29.1 (CH3) |
| 4 | 5 | ||||||
|---|---|---|---|---|---|---|---|
| δH | δC | HMBC | δH | δC | HMBC | ||
| 1 | 148.0 | 164.4 | |||||
| 2 | 139.6 | 6.20 br s | 98.4 | C-3, 9a, 4 | |||
| 3 | 7.34 s | 122.4 | C-2, 4a, 1′ | 165.5 | |||
| 4 | 126.1 | 6.40 br s | 94.3 | ||||
| 4a | 147.4 | 158.3 | |||||
| 5 | 129.6 | 132.1 | |||||
| 10a | 146.6 | 145.3 | |||||
| 6 | 154.0 | 150.4 | |||||
| 7 | 126.1 | 126.5 | |||||
| 8 | 7.79 s | 112.8 | C-9 | 7.51 s | 116.1 | C-9, 6, 10a, 1′ | |
| 8a | 115.2 | 113.4 | |||||
| 9 | 183.0 | 180.7 | |||||
| 9a | 109.0 | 102.8 | |||||
| 1′ | 40.5 | 3.43 d (6.6) | 29.1 | C-7, 2′, 3′ | |||
| 2′ | 6.36dd (17.7,10.8) | 147.8 | 5.41 t (6.6) | 122.4 | C-7, 1′, 4′, 5′ | ||
| 3′ | 5.16 d (17.7) | 110.9 | C-1′ | 136.8 | |||
| 5.02 d (10.8) | |||||||
| 4′ | 1.65 s | 27.3 | C-2′, 3′, 4 | 1.74 s | 15.9 | C-2′, 3′, 5′ | |
| 5′ | 1.65 s | 27.2 | C-2′, 3′, 4 | 2.08 m | 40.2 | C-2′, 3′, 2′′ | |
| 1′′ | 5.53 d (3.9) | 72.4 | 2.12 m | 28.3 | C-2′′, 3′′ | ||
| 2′′ | 4.49 d (3.9) | 99.8 | 5.13 t (6.0) | 124.7 | C-5′, 1′′, 4′′, 5′′ | ||
| 3′′ | 70.9 | 131.5 | |||||
| 4′′ | 1.32 s | 25.2 | C-2′′, 3′′ | 1.63 s | 25.5 | C-2′′, 3′′, 5′′ | |
| 5′′ | 1.30 s | 25.5 | C-2′′, 3′′ | 1.58 s | 17.4 | C-2′′, 3′′, 5′′ | |
| 1-OH | 13.0 s | C-2 | 13.22 s | ||||
2.2. DPPH radical-scavenging activities of the purified compounds
| Compound | DPPH radical-scavenging activity (IC50. μM ) |
|---|---|
| 1 | 19.64 ± 0.39 |
| 2 | 31.82 ± 0.08 |
| 3 | 22.07 ± 0.25 |
| 4 | 40.70 ± 0.10 |
| 5 | 34.27 ± 0.25 |
| 6 | 66.88 ± 0.19 |
| ascorbic acid | 13.16 ± 0.03 |
| gallic acid | 5.86 ± 0.03 |
3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and isolation procedures
3.4. Physical data of new compounds
3.5. DPPH radical scavenging activity
3.6. Statistical analyses of results of activity studies
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Hail, N., Jr.; Cortes, M.; Drake, E.N.; Spallholz, J.E. Cancer chemoprevention: A radical perspective. Free Radic. Biol. Med. 2008, 45, 97–110. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Spencer, J.P.E.; Warren, D.; Jenner, P.; Butler, J.; Halliwell, B. Characterization of food antioxidants, illustrated using commercial garlic and ginger preparations. Food Chem. 1997, 60, 149–156. [Google Scholar] [CrossRef]
- Ningappa, M.B.; Dinesha, R.; Srinivas, L. Antioxidant and free radical scavenging activities of polyphenol-enriched curry leaf (Murraya koenigii L.) extract. Food Chem. 2008, 106, 720–728. [Google Scholar] [CrossRef]
- Wang, B.G.; Zhang, W.W.; Duan, X.J.; Li, X.M. In vitro antioxidative activities of extract and semi-purified fractions of the marine red alga, Rhodomela confervoides (Rhodomelaceae). Food Chem. 2009, 113, 1101–1105. [Google Scholar] [CrossRef]
- Mbwambo, Z.H.; Kapingu, M.C.; Moshi, M.J.; Machumi, F.; Apers, S.; Cos, P.; Ferreira, D.; Marais, J.P.; Vanden Berghe, D.; Maes, L.; Vietinck, A.; Pieters, L. Antiparasitic activity of some xanthones and biflavonoids from the root bark of Garcinia livingstonei. J. Nat. Prod. 2006, 69, 369–372. [Google Scholar] [CrossRef]
- Peres, V.; Nagem, T.J.; Oliveira, D.F.F. Tetraoxygenated naturally occurring xanthones. Phytochemistry 2000, 55, 683–710. [Google Scholar]
- Franklin, G.; Conceicao, L.F.R.; Kombrink, E.; Dias, A.C.P. Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress. Phytochemistry 2009, 70, 60–68. [Google Scholar]
- Lin, Y.F.; Zhuan, Y.; Zhao, Y.H. Chinese Dai Medicine Colorful Illustrations; Yunnan National Publishing House: Kunming, China, 2003; p. 6, in Chinese. [Google Scholar]
- Karanjgoakar, C.G.; Rao, A.V.R.; Venkataraman, K.; Yemul, S.S.; Palmer, K.J. The constitution of xanthochymol and isoxanthochymol. Tetrahedron Lett. 1973, 50, 4977–4980. [Google Scholar]
- Blount, J.F.; Williams, T.H. Revised structure of xanthochymol. Tetrahedron Lett. 1976, 34, 2921–2924. [Google Scholar]
- Basa, S.C.; Mahanty, P.; Das, D.P. Isoxanthochymol: a revised structure. Chem. Ind. 1978, 4, 166–167. [Google Scholar]
- Tandon, R.N.; Srivastava, O.P.; Baslas, R.K.; Kumar, P. Preliminary investigation of the antimicrobial activity of a phytochemical, xanthochymol from the fruits of Garcinia xanthochymus Hook. F. Curr. Sci. 1980, 49, 472–473. [Google Scholar]
- Rao, A.V.R.; Venkatswamy, G.; Yemul, S.S. Xanthochymol & isoxanthochymol, two novel polyisoprenylated benzophenones from Garcinia xanthochymus. Indian J. Chem., Sect. B. 1980, 19, 627–633. [Google Scholar]
- Baslas, R.K.; Kumar, P. Isolation and characteristation of biflavnone and xanthones in the fruits of Garcinia xanthochymus. Acta Cienc. Indica 1981, 7, 31–34. [Google Scholar]
- Baggett, S.; Protiva, P.; Mazzola, E.P.; Yang, H.; Ressler, E.T.; Basile, M.J.; Bernard, W.; Edward, J.K. Bioactive benzophenones from Garcinia xanthochymus Fruits. J. Nat. Prod. 2005, 68, 354–360. [Google Scholar] [CrossRef]
- Konoshima, M.; Ikeshiro, Y.; Miyahara, S.; Yen, K.Y. The constitution of biflavonoids from Garcinia plants. Tetrahedron Lett. 1970, 48, 4203–4206. [Google Scholar]
- Baslas, R.K.; Kumar, P. Chemical examination of the fruits of Garcinia xanthochymus. Curr. Sci. 1979, 48, 814–815. [Google Scholar]
- Singh, M.P.; Parveen, N.; Khan, N.; Achari, B.; Dutta, P. Constituents of Garcinia xanthochymus. Fitoterapia 1991, 62, 286–289. [Google Scholar]
- Chanmahasathien, W.; Li, Y.S.; Satake, M.; Oshima, Y.; Ruangrungsi, N.; Ohizumi, Y. Prenylated xanthones with NGF-potentiating activity from Garcinia xanthochymus. Phytochemistry 2003, 64, 981–986. [Google Scholar] [CrossRef]
- Chanmahasathien, W.; Li, Y.S.; Satake, M.; Oshima, Y.; Ishibashi, M.; Ruangrungsi, N.; Ohizumi, Y. Prenylated xanthones from Garcinia xanthochymus. Chem. Pharm. Bull. 2003, 51, 1332–1334. [Google Scholar] [CrossRef]
- Han, Q.B.; Qiao, C.F.; Song, J.Z.; Yang, N.Y.; Cao, X.W.; Peng, Y.; Yang, D.J.; Chen, S.L.; Xu, H.X. Cytotoxic prenylated phenolic compounds from the twig bark of Garcinia xanthochymus. Chem. Biodivers. 2007, 4, 940–946. [Google Scholar] [CrossRef]
- Iinuma, M.; Tosa, H.; Tanaka, T.; Asai, F.; Shimano, R. Two new xanthone from the root bark of Garcinia subelliptica. Heterocycles 1995, 40, 279–284. [Google Scholar] [CrossRef]
- Hay, A.E.; Aumond, M.C.; Mallet, S.; Dumontet, V.; Litaudon, M.; Rondeau, D.; Richomme, P. Antioxidant xantones from Garcinia vieillardii. J. Nat. Prod. 2004, 67, 707–709. [Google Scholar] [CrossRef]
- Pinedo, A.T.D.; Penalver, P.; Morales, J.C. Synthesis and evaluation of new phrnolic-based antioxidants: Structure-activity relationship. Food Chem. 2007, 103, 55–61. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Alma, M.H.; Mavi, A.; Yilderim, A.; Digrak, M.; Hirata, T. Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in Turkey. Biol. Pharm. Bull. 2003, 26, 1725–1729. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.; Fan, H.; Yang, G.-z.; Jiang, Y.; Zhong, F.-f.; He, H.-w. Prenylated Xanthones from the Bark of Garcinia xanthochymus and Their 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activities. Molecules 2010, 15, 7438-7449. https://doi.org/10.3390/molecules15107438
Chen Y, Fan H, Yang G-z, Jiang Y, Zhong F-f, He H-w. Prenylated Xanthones from the Bark of Garcinia xanthochymus and Their 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activities. Molecules. 2010; 15(10):7438-7449. https://doi.org/10.3390/molecules15107438
Chicago/Turabian StyleChen, Yu, Hua Fan, Guang-zhong Yang, Yan Jiang, Fang-fang Zhong, and Hong-wu He. 2010. "Prenylated Xanthones from the Bark of Garcinia xanthochymus and Their 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activities" Molecules 15, no. 10: 7438-7449. https://doi.org/10.3390/molecules15107438




