The Apoptotic Effect of 1’S-1’-Acetoxychavicol Acetate from Alpinia Conchigera on Human Cancer Cells
Abstract
:1. Introduction
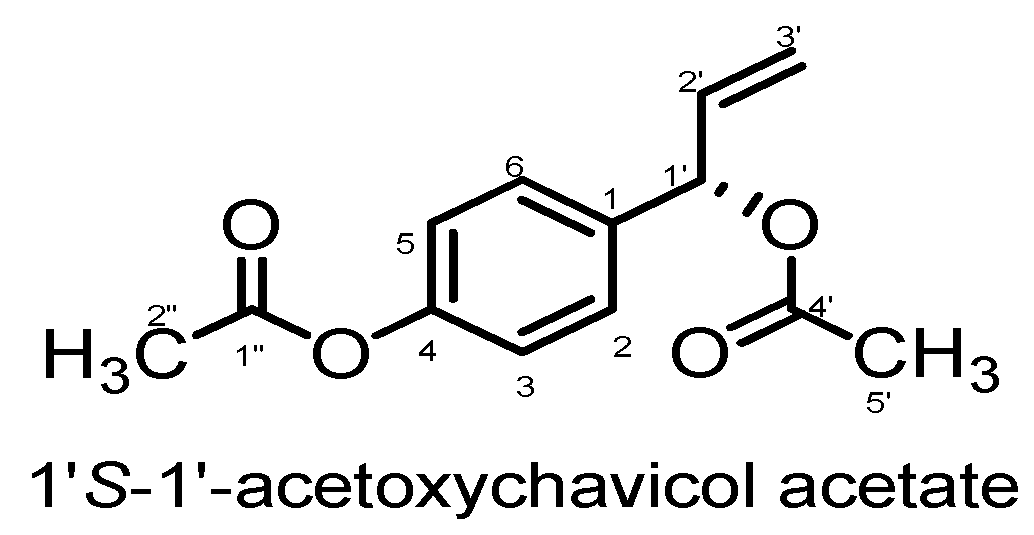
2. Results and Discussions
2.1. LC-MS Analysis

2.2. ACA Induced Dose- and Time-Dependent Cytotoxic in Tumour Cells
| Human Cancer Cell Lines | IC50 value (µM) | Incubation Time (hours) |
|---|---|---|
| Breast adenocarcinoma (MCF-7) | 34.0* | 12* |
| 30.0 | 24 | |
| Oral squamous carcinoma (HSC-2) | 9.0* | 12* |
| 5.0 | 24 | |
| Oral squamous carcinoma (HSC-4) | 8.0* | 12* |
| 5.5 | 24 | |
| Hepatocyte carcinoma (HepG2) | 48.0* | 12* |
| 18.0 | 24 | |
| Epidermoid cervical carcinoma (CaSki) | 48.0* | 12* |
| 17.0 | 24 |

2.3. ACA Induced-Potential Cell Cycle Arrest at the G0/G1 Phase
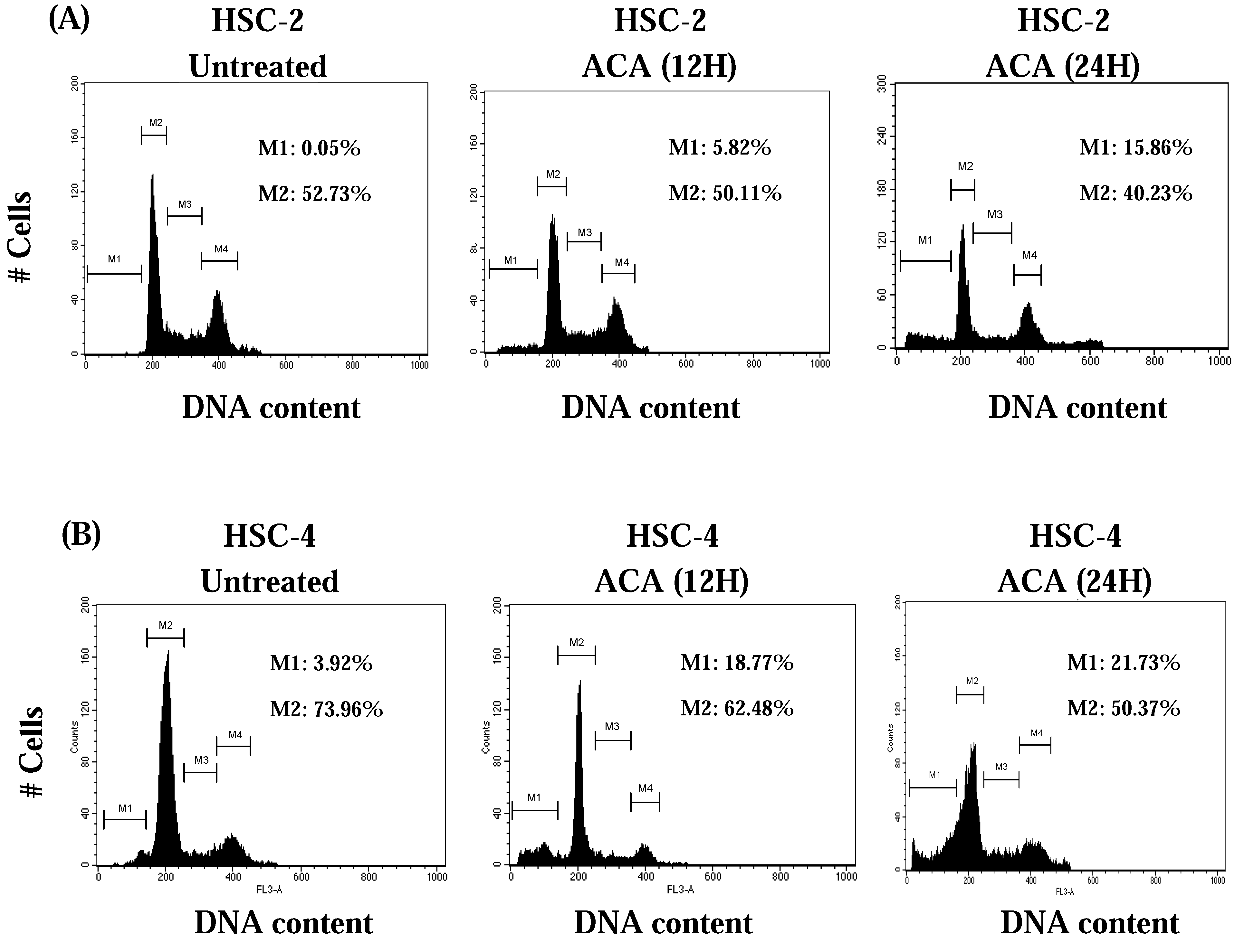
2.4. ACA Induced Cell Death via Apoptosis


3. Experimental
3.1. General
3.2. Chemicals
3.3. Plant Materials
3.4. Extraction and Isolation
3.5. 1’-(S)-1’-Acetoxychavicol Acetate:
3.6. LCMS Analysis
3.7. Cultivation of Cells
3.8. Cell Viability Assay
3.9. Cell Cycle Analysis
3.10. Annexin-V Apoptosis Assays
3.11. DNA Fragmentation Assays
3.12. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Will, R.B.H.; Bone, K.; Morgan, M. Herbal products: Active constituents, mode of action and quality control. Nutr. Res. Rev. 2000, 13, 47–77. [Google Scholar] [CrossRef]
- Itharat, A.; Houghton, P.J.; Eno-Amooquaye, E.; Burke, P.J.; Sampson, J.H.; Raman, A. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J. Ethnopharmacol. 2004, 90, 33–38. [Google Scholar] [CrossRef]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula, 2nd ed; Ministry of Agriculture and Cooperative: Kuala, Lumpur, Malaysia, 1966. [Google Scholar]
- Janssen, A.M.; Scheffer, J.C. Acetoxychavicol acetate, an antifungal component of Alpinia galanga. Planta Medica 1985, 507–511. [Google Scholar]
- Kress, W.J.; Liu, A.Z.; Newman, M.; Li, Q.J. The molecular phytogeny of Alpinia (Zingiberaceae): A complex and polyphyletic genus of gingers. Am. J. Bot. 2005, 92, 167–178. [Google Scholar] [CrossRef]
- Smith, R.M. Alpinia (Zingiberaceae): A Proposed New Infragenesis Classification; Notes of the Royal Botanic Garden: Edinburgh, UK, 1990; Volume 47, pp. 1–75. [Google Scholar]
- Larsen, K.; Ibrahim, H.; Khaw, S.H.; Saw, L.G. Gingers of Peninsular Malaysia and Singapore; Kota Kinabalu: Natural History Publications: Borneo, Malaysia, 1999. [Google Scholar]
- Ibrahim, H.; Chooi, O.H.; Hassan, R. Ethnobotanical survey of the ginger family in selected Malay villages in Peninsular Malaysia. Malaysian J. Sci. 2000, 24, 93–96. [Google Scholar]
- Matsuda, H.; Ando, S.; Morikawa, T.; Kataoka, S.; Yoshikawa, M. Structure-activity relationships of 1’S-1’-acetoxychavicol acetate for inhibitory effect on NO production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem. Lett. 2005, 15, 1949–1953. [Google Scholar] [CrossRef]
- Kondo, A.; Ohigashi, H.; Murakami, A.; Suratwadee, J.; Koshimizu, K. 1'-acetoxychavicol acetate as a potent inhibitor of tumor promoter-induced Epstein-Barr Virus activation from Languas galanga, a traditional thai condiment. Biosci. Biotech. Biochem. 1993, 57, 1344–1345. [Google Scholar] [CrossRef]
- Murakami, A.; Ohura, S.; Nakamura, Y.; Koshimizu, K.; Ohigashi, H. 1’-acetoxychavicol acetate, a superoxide anion generation inhibitor, potently inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in ICR mouse skin. Oncology 1996, 53, 386–391. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawabata, K.; Kakumoto, M.; Makita, H.; Matsunaga, K.; Mori, H.; Satoh, K.; Hara, A.; Murakami, A.; Koshimizu, K.; Ohigashi, H. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by a xanthine oxidase inhibitor 1’-acetoxychavicol acetate. Jpn. J. Cancer Res. 1997, 88, 821–830. [Google Scholar] [CrossRef]
- Tanaka, T.; Makita, H.; Kawamori, T.; Kawabata, K.; Mori, H.; Murakami, A.; Satoh, K.; Hara, A.; Ohigashi, H.; Koshimizu, K. 1’-acetoxychavicol acetate inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Carcinogenesis 1997, 18, 1113–1118. [Google Scholar] [CrossRef]
- Nakamura, A.; Murakami, Y.; Ohto, K.; Torikai, T.; Tanaka, T.; Ohigashi, H. Suppresion of tumor promoter-induced oxidative stress and inflammatory responses in mouse skin by superoxide generation inhibitor 1'-acetoxychavicol acetate. Cancer Res. 1998, 58, 4832–4839. [Google Scholar]
- Ito, K.; Nakazato, T.; Murakami, A.; Yamato, K.; Miyakawa, Y.; Yamada, T.; Hozumi, N.; Ohigashi, H.; Ikeda, Y.; Kizaki, M. Induction of apoptosis in human myeloid leukemic cells by 1’-acetoxychavicol acetate through a mitochondrial- and Fas-mediated dual mechanism. Clin. Cancer. Res. 2004, 10, 2120–2130. [Google Scholar]
- Voilante, G.D.; Zerrouk, N.; Richard, I.; Provot, G.; Chaumil, J.C.; Arnoud, P. Evaluation of the cytotoxicity effect of dimethyl sulfoxide (DMSO) on Caco21TC7 colon tumor cell cultures. Biol. Pharm. Bull. 2002, 25, 1600–1603. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Apoptosis, oncosis and necrosis: An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar]
- Renehan, A.G.; Booth, C.; Potten, C.S. What is apoptosis, and why is it important? BMJ 2001, 322, 1536–1538. [Google Scholar]
- Ito, K.; Nakazato, T.; Xian, M.J.; Yamada, T.; Hozumi, N.; Murakami, A.; Ohigashi, H.; Ikeda, Y.; Kizaki, M. 1’-acetoxychavicol acetate is a novel Nuclear Factor κB inhibitor with significant activity against multiple myeloma in vitro and in vivo. Cancer Res. 2005, 65, 4417–4424. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Wyllie, A.H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980, 284, 555–556. [Google Scholar] [CrossRef]
- Mitsui, S.; Kobayashi, S.; Nayahori, H.; Ogiso, A. Constituents from seeds of Alpinia galanga Wild and their anti-ulcer activities. Chem. Pharm. Bull 1976, 24, 2377–2382. [Google Scholar] [CrossRef]
- Barik, B.R.; Kundu, A.B.; Dey, A.K. Two phenolic constituents from Alpinia galanga rhizomes. Phytochemistry 1987, 26, 2126–2127. [Google Scholar] [CrossRef]
- Yang, X.; Eilerman, R. G. Purgent principle of Alpinia galanga (L.) Swartz and its application. J. Agr. Food Chem. 1999, 47, 1657–1662. [Google Scholar] [CrossRef]
- Lee, S.J.; Ando, T. Optically active 1’-acetoxychavicol acetate and its positional isomers: Synthesis repellent effect against adzuki bean weevil. J. Pesticide Sci. 2001, 26, 76–81. [Google Scholar] [CrossRef]
- Ando, S.; Matsuda, H.; Morikawa, T.; Yoshikawa, M. 1’S-1’-acetoxychavicol acetate as a new type inhibitor of interferon-β production in lipopolisaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem. 2005, 13, 3289–3294. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Awang, K.; Nurul Azmi, M.N.; In Lian Aun, L.I.L.; Nazif Aziz, A.N.; Ibrahim, H.; Hasima Nagoor, N. The Apoptotic Effect of 1’S-1’-Acetoxychavicol Acetate from Alpinia Conchigera on Human Cancer Cells. Molecules 2010, 15, 8048-8059. https://doi.org/10.3390/molecules15118048
Awang K, Nurul Azmi MN, In Lian Aun LIL, Nazif Aziz AN, Ibrahim H, Hasima Nagoor N. The Apoptotic Effect of 1’S-1’-Acetoxychavicol Acetate from Alpinia Conchigera on Human Cancer Cells. Molecules. 2010; 15(11):8048-8059. https://doi.org/10.3390/molecules15118048
Chicago/Turabian StyleAwang, Khalijah, Mohamad Nurul Nurul Azmi, Lionel In Lian In Lian Aun, Ahmad Nazif Nazif Aziz, Halijah Ibrahim, and Noor Hasima Nagoor. 2010. "The Apoptotic Effect of 1’S-1’-Acetoxychavicol Acetate from Alpinia Conchigera on Human Cancer Cells" Molecules 15, no. 11: 8048-8059. https://doi.org/10.3390/molecules15118048




