Synthesis, Antimycobacterial, Antifungal and Photosynthesis-Inhibiting Activity of Chlorinated N-phenylpyrazine-2-carboxamides †
Abstract
:1. Introduction

2. Results and Discussion
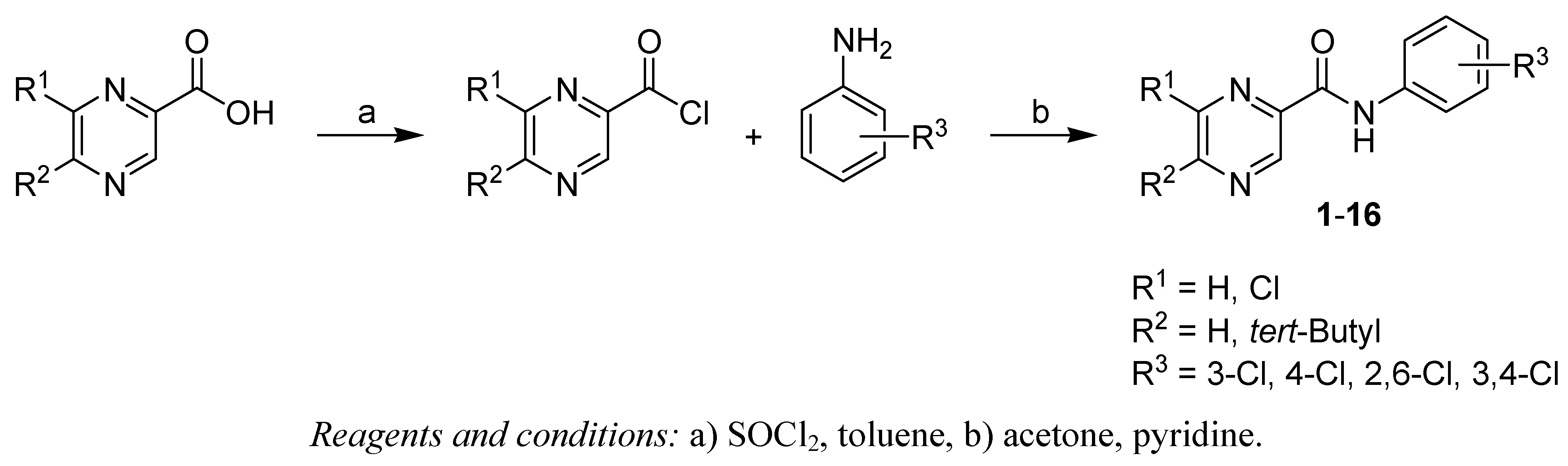
2.1. Chemistry
2.2. Lipophilicity

| Comp. | R1 | R2 | R3 | log k | log P ACD/LogP | πdetermined Pyr/Ph | σ [15,16] |
|---|---|---|---|---|---|---|---|
| 1 | H | H | 3-Cl | 0.4914 | 2.17 ± 0.41 | 0.00/0.08 | 0.373 |
| 2 | Cl | H | 3-Cl | 0.7864 | 3.29 ± 0.42 | 0.30/0.10 | 0.373 |
| 3 | H | (CH3)3C | 3-Cl | 1.0996 | 3.85 ± 0.41 | 0.61/0.26 | 0.373 |
| 4 | Cl | (CH3)3C | 3-Cl | 1.4896 | 4.98 ± 0.43 | 1.00/0.25 | 0.373 |
| 5 | H | H | 4-Cl | 0.4987 | 2.13 ± 0.41 | 0.00/0.09 | 0.227 |
| 6 | Cl | H | 4-Cl | 0.8185 | 3.25 ± 0.42 | 0.32/0.13 | 0.227 |
| 7 | H | (CH3)3C | 4-Cl | 1.1043 | 3.81 ± 0.41 | 0.61/0.16 | 0.227 |
| 8 | Cl | (CH3)3C | 4-Cl | 1.5015 | 4.91 ± 0.43 | 1.00/0.26 | 0.227 |
| 9 | H | H | 2,6-Cl | 0.6656 | 2.17 ± 0.41 | 0.00/0.25 | 0.40 |
| 10 | Cl | H | 2,6-Cl | 0.9456 | 3.29 ± 0.43 | 0.30/0.28 | 0.40 |
| 11 | H | (CH3)3C | 2,6-Cl | 1.2802 | 3.85 ± 0.42 | 0.61/0.34 | 0.40 |
| 12 | Cl | (CH3)3C | 2,6-Cl | 1.6631 | 4.97 ± 0.44 | 1.00/0.42 | 0.40 |
| 13 | H | H | 3,4-Cl | 0.6962 | 3.03 ± 0.42 | 0.00/0.30 | 0.60 |
| 14 | Cl | H | 3,4-Cl | 0.9950 | 4.15 ± 0.44 | 0.28/0.31 | 0.60 |
| 15 | H | (CH3)3C | 3,4-Cl | 1.3395 | 4.72 ± 0.43 | 0.62/0.40 | 0.60 |
| 16 | Cl | (CH3)3C | 3,4-Cl | 1.7563 | 5.84 ± 0.45 | 1.04/0.51 | 0.60 |

2.3. In vitro antimycobacterial evaluation
| Comp. | Antimycobacterial evaluation Inhibition [%] | Antifungal susceptibility MIC [µmol/L] a | PET inhibitionIC50 [μmol/L] |
|---|---|---|---|
| 1 | 14 | 500/>500 | 290.1 |
| 2 | 14 | 125/125 | 262.0 |
| 3 | 0 | >250/>250 | 95.5 |
| 4 | 0 | >250/>250 | 100.7 |
| 5 | 4 | >250/>250 | 1,523 |
| 6 | 65 | >500/>500 | 486.0 |
| 7 | 0 | >250/>250 | ND |
| 8 | 24 | >250/>250 | 43.0 |
| 9 | 0 | >500/>500 | ND |
| 10 | 0 | >250/>250 | 829.3 |
| 11 | 0 | 250/250 | 153.0 |
| 12 | 0 | 125/125 | 61.0 |
| 13 | 8 | >250/>250 | ND |
| 14 | 61 | 125/250 | 104.8 |
| 15 | 15 | 125/125 | ND |
| 16 | 0 | 62.5/62.5 | 130.1 |
| PZA | b | – | – |
| FLU | – | 1.95/3.91 | – |
| DCMU | – | – | 1.9 |
2.4. In vitro antifungal susceptibility testing
2.5. Inhibition of photosynthetic electron transport (PET) in spinach chloroplasts
3. Experimental
3.1. General
3.2. Synthesis
3.2.1. General procedure for the synthesis of compounds 1-16
3.3. Lipophilicity determination by HPLC (capacity factor k/calculated log k)
3.4. Lipophilicity calculations
3.5. In vitro antimycobacterial screening
3.6. In vitro antifungal susceptibility testing
3.7. Study of inhibition photosynthetic electron transport (PET) in spinach chloroplasts
4. Conclusions
Acknowledgements
- Samples Availability: Samples of the compounds are available from the authors.
References
- Dlabal, K.; Doležal, M.; Macháček, M. Preparation of some 6-substituted N-pyrazinyl-2-pyrazinecarboxamides. Collect. Czech. Chem. Commun. 1993, 58, 452–454. [Google Scholar] [CrossRef]
- Doležal, M.; Vičík, R.; Miletín, M.; Kráľová, K. Synthesis and antimycobacterial, antifungal, and photosynthesis-inhibiting evaluation of some anilides of substituted pyrazine-2-carboxylic acids. Chem. Pap. 2000, 54, 245–248. [Google Scholar]
- Doležal, M.; Miletín, M.; Kuneš, J.; Kráľová, K. Substituted amides of pyrazine-2-carboxylic acids, their synthesis and biological activity. Molecules 2002, 7, 363–373. [Google Scholar] [CrossRef]
- Doležal, M.; Palek, L.; Vinšová, J.; Buchta, V.; Jampílek, J.; Kráľová, K. Substituted pyrazinecarboxamides: Synthesis and their biological evaluation. Molecules 2006, 11, 242–256. [Google Scholar] [CrossRef]
- Doležal, M.; Čmedlová, P.; Palek, L.; Vinšová, J.; Kuneš, J.; Buchta, V.; Jampílek, J.; Kráľová, K. Synthesis and biological evaluation of pyrazinecarboxamides. Eur. J. Med. Chem. 2008, 43, 1105–1113. [Google Scholar] [CrossRef]
- Doležal, M.; Zitko, J.; Kešetovičová, D.; Kuneš, J.; Svobodová, M. Substituted N-phenylpyrazine-2-carboxamides: Synthesis and antimycobacterial evaluation. Molecules 2009, 14, 4180–4189. [Google Scholar] [CrossRef]
- Doležal, M.; Hartl, J.; Miletín, M.; Macháček, M.; Kráľová, K. Synthesis and photosynthesis-inhibiting activity of some anilides of substituted pyrazine-2-carboxylic acids. Chem. Pap. 1999, 53, 126–130. [Google Scholar]
- Doležal, M.; Kráľová, K.; Šeršeň, F.; Miletín, M. The site of action of some anilides of pyrazine-2-carboxylic acids in the photosynthetic apparatus. Folia Pharm. Univ. Carol. 2001, 26, 13–20. [Google Scholar]
- Tůmová, L.; Gallová, K.; Řimáková, J.; Doležal, M.; Tůma, J. The effect of substituted amides of pyrazine-2-carboxylic acids on flavonolignan production in Silybum marianum culture in vitro. Acta Physiol. Plant. 2005, 27, 357–362. [Google Scholar]
- Doležal, M.; Tůmová, L.; Kešetovičová, D.; Tůma, J.; Kráľová, K. Substituted N-phenylpyrazine-2-carboxamides, their synthesis and evaluation as herbicides and abiotic elicitors. Molecules 2007, 12, 2589–2598. [Google Scholar] [CrossRef]
- Tůmová, L.; Tůma, J.; Megušar, K.; Doležal, M. Substituted pyrazinecarboxamides as abiotic elicitors of flavolignan production in Silybum marianum (L.) gaertn cultures in vitro. Molecules 2010, 15, 331–340. [Google Scholar]
- Stancheva, I.; Georgiev, G.; Geneva, M.; Ivanova, A; Doležal, M.; Tůmová, L. Influence of foliar fertilization and growth effector 5-tert-butyl-N-m-tolylpyrazine-2-carboxamide (MD 148/II) on the milk thistle (Silybum marianum L.) seed yield and quality. J. Plant Nutr. 2010, 33, 818–830. [Google Scholar]
- Kerns, E.H.; Li, D. Drug-like Properties: Concept, Structure Design and Methods; Elsevier: San Diego, CA, USA, 2008; pp. 122–136. [Google Scholar]
- Hansch, C.; Leo, A.; Unger, S.H.; Nikaitani, D.; Lien, E.J. Aromatic substituent constant for structure–activity correlations. J. Med. Chem. 1973, 16, 1207–1216. [Google Scholar] [CrossRef]
- Norrington, F.E.; Hyde, R.M.; Williams, S.G.; Wotton, R. Physicochemical-activity relations in practice. 1. Rational and self-consistent data bank. J. Med. Chem. 1975, 18, 604–607. [Google Scholar]
- Fujita, T.; Nishioka, T. The analysis of the ortho effect. Prog. Phys. Org. Chem. 1976, 12, 49–89. [Google Scholar] [CrossRef]
- TAACF. Global discovery program for novel anti-tuberculosis drugs. Available online: http://www.taacf.org/about-TAACF.htm accessed on 10 October 2010.
- Doležal, M.; Jampílek, J.; Osička, Z.; Kuneš, J.; Buchta, V.; Víchová, P. Substituted 5-aroylpyrazine-2-carboxylic acid derivatives: Synthesis and biological activity. Farmaco 2003, 58, 1105–1111. [Google Scholar] [CrossRef]
- Kráľová, K.; Šeršeň, F.; Miletín, M.; Doležal, M. Inhibition of photosynthetic electron transport in spinach chloroplasts by 2,6-disubstituted pyridine-4-thiocarboxamides. Chem. Pap. 2002, 56, 214–217. [Google Scholar]
- Draber, W.; Tietjen, K.; Kluth, J.F.; Trebst, A. Herbicides in photosynthesis research. Angew. Chem. 1991, 3, 1621–1633. [Google Scholar]
- Tischer, W.; Strotmann, H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron-transport. Biochim. Biophys. Acta 1977, 460, 113–125. [Google Scholar] [CrossRef]
- Trebst, A.; Draber, W. Structure activity correlations of recent herbicides in photosynthetic reactions. In Advances in Pesticide Science; Greissbuehler, H., Ed.; Pergamon Press: Oxford, UK, 1979; pp. 223–234. [Google Scholar]
- Bowyer, J.R.; Camilleri, P.; Vermaas, W.F.J. Herbicides, Topics in Photosynthesis; Baker, N.R., Percival, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 10, pp. 27–85. [Google Scholar]
- Collins, L.A.; Franzblau, S.G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997, 41, 1004–1009. [Google Scholar]
- National Committee for Clinical Laboratory Standards, Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts: Approved Guideline M44-A; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2004.
- Masarovičová, E.; Kráľová, K. Approaches to measuring plant photosynthesis activity. In Handbook of Photosynthesis, 2nd; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 617–656. [Google Scholar]
- Kráľová, K.; Šeršeň, F.; Sidóová, E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992, 46, 348–350. [Google Scholar]
- Fedke, C. Biochemistry and Physiology of Herbicide Action; Springer Verlag: New York, NY, USA, 1982. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dolezal, M.; Zitko, J.; Osicka, Z.; Kunes, J.; Vejsova, M.; Buchta, V.; Dohnal, J.; Jampilek, J.; Kralova, K. Synthesis, Antimycobacterial, Antifungal and Photosynthesis-Inhibiting Activity of Chlorinated N-phenylpyrazine-2-carboxamides †. Molecules 2010, 15, 8567-8581. https://doi.org/10.3390/molecules15128567
Dolezal M, Zitko J, Osicka Z, Kunes J, Vejsova M, Buchta V, Dohnal J, Jampilek J, Kralova K. Synthesis, Antimycobacterial, Antifungal and Photosynthesis-Inhibiting Activity of Chlorinated N-phenylpyrazine-2-carboxamides †. Molecules. 2010; 15(12):8567-8581. https://doi.org/10.3390/molecules15128567
Chicago/Turabian StyleDolezal, Martin, Jan Zitko, Zdenek Osicka, Jiri Kunes, Marcela Vejsova, Vladimir Buchta, Jiri Dohnal, Josef Jampilek, and Katarina Kralova. 2010. "Synthesis, Antimycobacterial, Antifungal and Photosynthesis-Inhibiting Activity of Chlorinated N-phenylpyrazine-2-carboxamides †" Molecules 15, no. 12: 8567-8581. https://doi.org/10.3390/molecules15128567
APA StyleDolezal, M., Zitko, J., Osicka, Z., Kunes, J., Vejsova, M., Buchta, V., Dohnal, J., Jampilek, J., & Kralova, K. (2010). Synthesis, Antimycobacterial, Antifungal and Photosynthesis-Inhibiting Activity of Chlorinated N-phenylpyrazine-2-carboxamides †. Molecules, 15(12), 8567-8581. https://doi.org/10.3390/molecules15128567






