Platycoside N: A New Oleanane-Type Triterpenoid Saponin from the Roots of Platycodon grandiflorum
Abstract
:1. Introduction
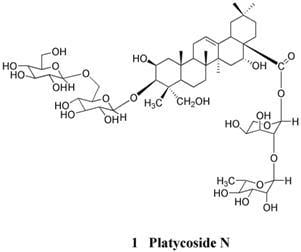
2. Results and Discussion
3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and isolation
3.4. Acid hydrolysis of 1
4. Conclusions
Acknowledgements
References and Notes
- Lee, K.J.; Kim, J.Y.; Jung, K.S.; Choi, C.Y.; Chung, Y.C.; Kim, D.H.; Jeong, H.G. Suppressive effects of Platycodon grandiflorum on the progress of carbon tetrachloride-induced hepatic fibrosis. Arch. Pharm. Res. 2004, 27, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Hwang, Y.P.; Lee, H.S.; Jeong, H.G. Inhibitory effect of Platycodi Radix on ovalbumin-induced airway inflammation in a murine model of asthma. Food Chem. Toxicol. 2009, 47, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Han, E.H.; Jin, Y.H.; Hwang, Y.P.; Kim, H.G.; Park, B.H.; Kim, J.Y.; Chung, Y.C.; Lee, K.Y.; Jeong, H.G. Saponins from the roots of Platycodon grandiflorum stimulate osteoblast differentiation via p38 MAPK- and ERK-dependent RUNX2 activation. Food Chem. Toxicol. 2010, 48, 3362–3368. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B. Pharmacological studies on Platycodon grandiflorum A. DC. IV. A comparison of experimental pharmacological effects of crude platycodin with clinical indications of platycodi radix. Yakugaku Zasshi 1973, 93, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Ezaki, O.; Ikemoto, S.; Itakura, H. Effects of Platycodon grandiflorum feeding on serum and liver lipid concentrations in rats with diet-induced hyperlipidemia. J. Nutr. Sci. Vitaminol. 1995, 41, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.Y.; Lee, W.J.; Lee, E.B.; Choi, E.Y.; Ko, K.H. Platycodin D and D3 increase airway mucin release in vivo and in vitro in rats and hamsters. Planta Med. 2002, 68, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.P.; Lee, E.B.; Kim, S.Y.; Li, D.; Ban, H.S.; Lim, S.S.; Shin, K.H.; Ohuchi, K. Inhibition of prostaglandin E2 production by platycodin D isolated from the root of Platycodon grandiflorum. Planta Med. 2001, 67, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.; Xu, B.J.; Kimura, Y.; Zheng, Y.N.; Okuda, H. Platycodi radix affects lipid metabolism in mice with high fat diet-induced obesity. J. Nutr. 2000, 130, 2760–2764. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.; Zheng, Y.N.; Xu, B.J.; Okuda, H.; Kimura, Y. Saponins from platycodi radix ameliorate high fat diet-induced obesity in mice. J. Nutr. 2002, 132, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Han, L.K.; Zheng, Y.N.; Lee, J.H.; Sung, C.K. In vitro inhibitory effect of triterpenoidal saponins from Platycodi Radix on pancreatic lipase. Arch. Pharm. Res. 2005, 28, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Kim, Y.S. Determination of the kinetic properties of platycodin D for the inhibition of pancreatic lipase using a 1,2-diglyceride-based colorimetric assay. Arch. Pharm. Res. 2004, 27, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.O.; Moon, D.O.; Choi, Y.H.; Shin, D.Y.; Kang, H.S.; Choi, B.T.; Lee, J.D.; Li, W.; Kim, G.Y. Platycodin D induces apoptosis and decreases telomerase activity in human leukemia cells. Cancer Lett. 2008, 261, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, K.W.; Moon, K.D.; Lee, M.K.; Choi, J.; Yee, S.T.; Shim, K.H.; Seo, K.I. Induction of apoptosis in HT-29 colon cancer cells by crude saponin from Platycodi Radix. Food Chem. Toxicol. 2008, 46, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Kim, G.Y.; Li, W.; Choi, B.T.; Kim, N.D.; Kang, H.S.; Choi, Y.H. Implication of intracellular ROS formation, caspase-3 activation and Egr-1 induction in platycodon D-induced apoptosis of U937 human leukemia cells. Biomed. Pharmacother. 2009, 63, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.H.; Tian, J.K. Cytotoxic triterpenoid saponins from the roots of Platycodon grandiflorum. Molecules 2007, 12, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, R.; Kim, Y.S.; Chung, S.I.; Yoon, Y. Platycodin D inhibits adipogenesis of 3T3-L1 cells by modulating kruppel-like factor 2 and peroxisome proliferator-activated receptor gamma. Phytother. Res. 2009, 24, S161–S167. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, J.; Ji, B.; Li, Y.; Zhang, X. Antihyperglycemic effects of Platycodon grandiflorum (Jacq.) A. DC. extract on streptozotocin-induced diabetic mice. Plant Foods Hum. Nutr. 2007, 62, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yoo, D.S.; Choi, C.W.; Cha, M.R.; Kim, Y.S.; Lee, H.S.; Lee, K.R.; Ryu, S.Y. Platyconic acid A, a genuine triterpenoid saponin from the roots of Platycodon grandiflorum. Molecules 2008, 13, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Dou, D.Q.; Zhao, C.J.; Shimizu, N.; Pei, Y.P.; Pei, Y.H.; Chen, Y.J.; Takeda, T. Triterpenoid saponins from Platycodon grandiflorum. J. Asian Nat. Prod. Res. 2007, 9, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Shimizu, N.; Dou, D.Q.; Takeda, T.; Fu, R.; Pei, Y.H.; Chen, Y.J. Five new triterpenoid saponins from the roots of Platycodon grandiflorum. Chem. Pharm. Bull 2006, 54, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Choi, S.U.; Kim, J.S.; Lee, H.S.; Roh, S.H.; Jeong, Y.C.; Kim, Y.K.; Ryu, S.Y. Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta Med. 2005, 71, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Shimizu, N.; Takeda, T.; Dou, D.Q.; Chen, B.; Pei, Y.H.; Chen, Y.J. New A-ring lactone triterpenoid saponins from the roots of Platycodon grandiflorum. Chem. Pharm. Bull. 2006, 54, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Hou, W.B.; Dou, D.Q.; Hua, H.M.; Gui, M.H.; Fu, R.; Chen, Y.J.; Pei, Y.H. Saponins of polygalacic acid type from Platycodon grandiflorum (in Chinese). Yao Xue Xue Bao 2006, 41, 358–360. [Google Scholar] [PubMed]
- Nikaido, T.; Koike, K.; Mitsunaga, K.; Saeki, T. Two new triterpenoid saponins from Platycodon grandiflorum. Chem. Pharm. Bull. 1999, 47, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiang, L.; Zhang, J.; Zheng, Y.N.; Han, L.K.; Saito, M. A New Triterpenoid Saponin from the Roots of Platycodon grandiflorum. Chin. Chem. Lett. 2007, 18, 306–308. [Google Scholar] [CrossRef]
- He, Z.; Qiao, C.; Han, Q.; Wang, Y.; Ye, W.; Xu, H. New triterpenoid saponins from the roots of Platycodon grandiflorum. Tetrahedron 2005, 61, 2211–2215. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors ([email protected]). |


| Position | δ C | Position | δ C |
|---|---|---|---|
| 1 | 46.8 | 3-O-Glc | |
| 2 | 70.1 | 1 | 106.7 |
| 3 | 83.1 | 2 | 75.3 |
| 4 | 42.4 | 3 | 78.7 |
| 5 | 47.5 | 4 | 72.1 |
| 6 | 20.5 | 5 | 76.0 |
| 7 | 33.6 | 6 | 70.8 |
| 8 | 40.2 | Glc | |
| 9 | 47.5 | 1′ | 106.7 |
| 10 | 37.2 | 2′ | 74.9 |
| 11 | 24.4 | 3′ | 78.7 |
| 12 | 123.1 | 4′ | 71.6 |
| 13 | 144.5 | 5′ | 78.7 |
| 14 | 42.4 | 6′ | 62.3 |
| 15 | 36.2 | Ara | |
| 16 | 74.2 | 1 | 93.9 |
| 17 | 48.6 | 2 | 75.3 |
| 18 | 41.5 | 3 | 70.8 |
| 19 | 47.2 | 4 | 66.6 |
| 20 | 31.0 | 5 | 63.7 |
| 21 | 36.2 | Rha | |
| 22 | 32.2 | 1 | 101.5 |
| 23 | 66.6 | 2 | 71.6 |
| 24 | 16.0 | 3 | 72.7 |
| 25 | 18.6 | 4 | 73.1 |
| 26 | 17.7 | 5 | 68.7 |
| 27 | 27.2 | 6 | 18.6 |
| 28 | 176.0 | ||
| 29 | 33.3 | ||
| 30 | 24.8 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, W.; Zhang, W.; Xiang, L.; Wang, Z.; Zheng, Y.-N.; Wang, Y.-P.; Zhang, J.; Chen, L. Platycoside N: A New Oleanane-Type Triterpenoid Saponin from the Roots of Platycodon grandiflorum. Molecules 2010, 15, 8702-8708. https://doi.org/10.3390/molecules15128702
Li W, Zhang W, Xiang L, Wang Z, Zheng Y-N, Wang Y-P, Zhang J, Chen L. Platycoside N: A New Oleanane-Type Triterpenoid Saponin from the Roots of Platycodon grandiflorum. Molecules. 2010; 15(12):8702-8708. https://doi.org/10.3390/molecules15128702
Chicago/Turabian StyleLi, Wei, Wei Zhang, Lan Xiang, Zi Wang, Yi-Nan Zheng, Ying-Ping Wang, Jing Zhang, and Li Chen. 2010. "Platycoside N: A New Oleanane-Type Triterpenoid Saponin from the Roots of Platycodon grandiflorum" Molecules 15, no. 12: 8702-8708. https://doi.org/10.3390/molecules15128702