Apoptotic Effects of γ-Mangostin from the Fruit Hull of Garcinia mangostana on Human Malignant Glioma Cells
Abstract
:1. Introduction
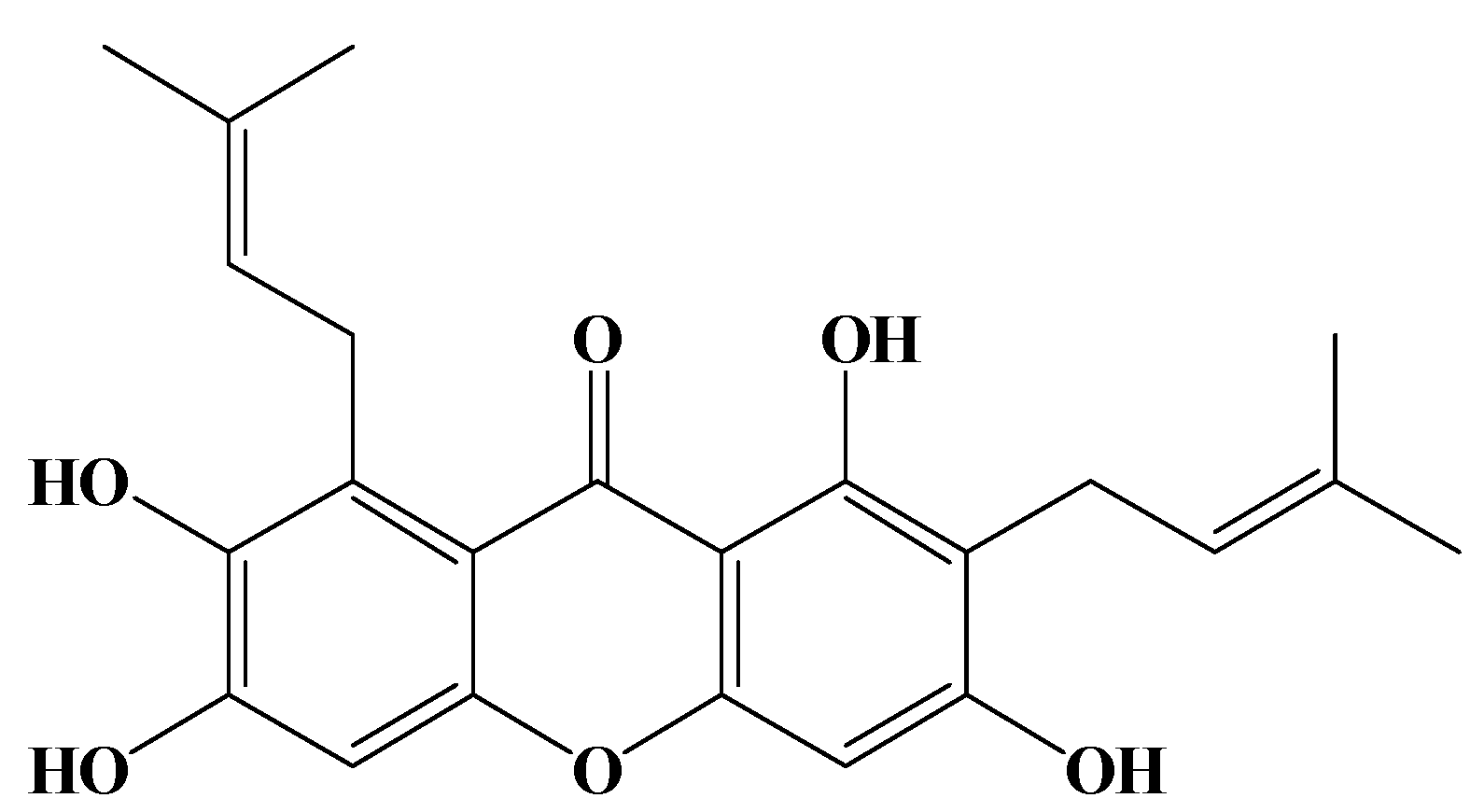
2. Results and Discussion
2.1. Antiproliferation of γ-mangostin in U87 MG and GBM 8401 glioma cells
| Drug (μM) | Cytotoxicity (%) | |
|---|---|---|
| U87 MG | GBM 8401 | |
| γ-mangostin | ||
| 10 | 0.00 ± 9.01 | 0.00 ± 0.00 |
| 20 | 0.00 ± 4.79 | 11.97 ± 3.12 |
| 40 | 3.64 ± 5.28 | 22.79 ± 6.20 |
| 80 | 55.76 ± 7.38 | 65.58 ± 0.43 |
| 100 | 89.18 ± 3.79 | 76.18 ± 7.14 |
| 200 | 98.83 ± 0.27 | 99.34 ± 0.13 |
| BCNUa | ||
| 934 | 71.47 ± 5.49 | 92.30 ± 2.50 |
| Cell line | IC50 (μM)a | Cytotoxicity (%) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| U87 MG | 74.14 ± 2.93 | 59.89 ± 0.03 | 73.78 ± 0.30 | 90.25 ± 2.71 |
| GBM 8401 | 64.67 ± 2.42 | 54.22 ± 0.04 | 60.92 ± 0.01 | 99.08 ± 0.36 |
2.2. γ-Mangostin induces the apoptosis process via intracellular ROS production
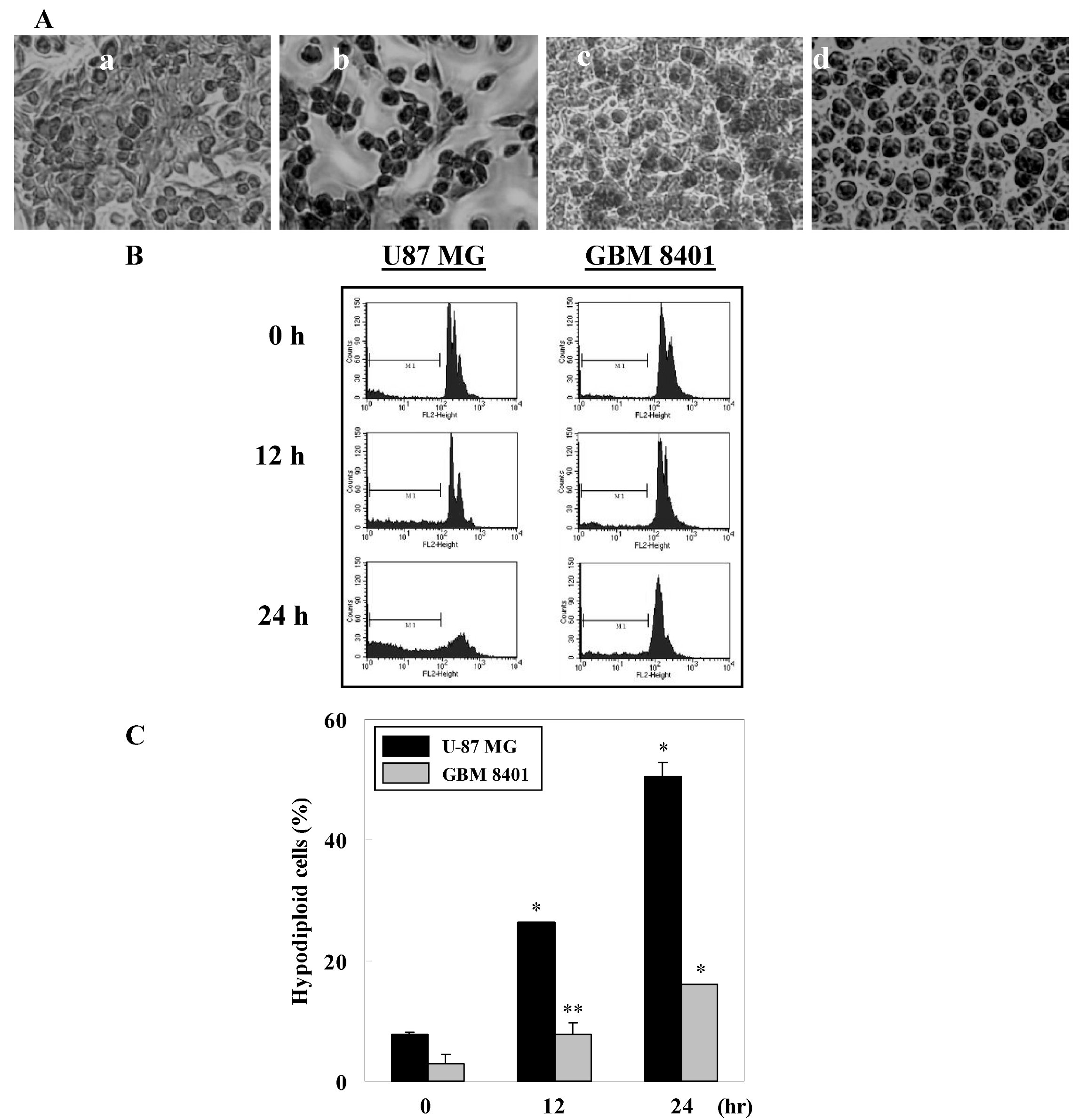


2.3. Cell damage and ROS-dependent mitochondrial dysfunction


3. Experimental
3.1. Chemicals
3.2. Extraction, isolation, and purification of γ-mangostin
3.3. Cell lines and cell culture
3.4. Animals
3.5. Preparation of rat brain mitochondria
3.6. Lactate dehydrogenase (LDH) assay
3.7. Thiobarbituric acid reactive substances (TBARS) and malondialdehyde (MDA) assay
3.8. Cell viability assays
3.9. Morphological study using fluorescence microscopy
3.10. Apoptosis and intracellular ROS determination
3.11. Detection of the mitochondrial function
3.12. Statistical analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of γ-mangostin are available from the corresponding author.
References and Notes
- Yukihiro, A.; Yoshihito, N.; Munekazu, I.; Yoshinori, N. Anti-cancer effects of xanthones from pericarps of mangosteen. Int. J. Mol. Sci. 2008, 9, 355–370. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akao, Y.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorg. Med. Chem. 2005, 13, 6064–6069. [Google Scholar] [CrossRef]
- Hitoshi, D.; Sibata, M.; Shibata, E.; Morimoto, J.; Akao, Y.; Iinuma, M.; Tanigawa, N.; Otsuki, Y. Panaxanthone isolated from pericarp of Garcinia mangostana L. suppresses tumor growth and metastasis of a mouse model of mammary cancer. Anticancer Res. 2009, 29, 2485–2496. [Google Scholar]
- Riese-Jorda, H.H.; Baez, J.M. Pharmacogenomics in neurooncology. Rev. Neurol. 2006, 43, 353–356. [Google Scholar]
- La Rocca, R.V.; Mehdorn, H.M. Localized BCNU chemotherapy and the multimodal management of malignant glioma. Curr. Med. Res. Opin. 2009, 25, 149–160. [Google Scholar] [CrossRef]
- Takvorian, T.; Parker, L.M.; Hochberg, F.H.; Canellos, G.P. Autologous bone-marrow transplantation: Host effects of high-dose BCNU. J. Clin. Oncol. 1983, 1, 610–620. [Google Scholar]
- Lee, H.H.; Yang, L.L.; Wang, C.C.; Hu, S.Y.; Chang, S.F.; Lee, Y.H. Differential effects of natural polyphenols on neuronal survival in primary cultured central neurons against glutamate and glucose deprivation induced neuronal death. Brain Res. 2003, 986, 103–113. [Google Scholar] [CrossRef]
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef]
- Lee, W.H.; Jin, J.S.; Tsai, W.C.; Chen, Y.T.; Chang, W.L.; Yao, C.W.; Sheu, L.F.; Chen, A. Biological inhibitory effects of the Chinese herb danggui on brain astrocytoma. Pathobiology 2006, 73, 141–148. [Google Scholar] [CrossRef]
- Lin, J.; Chen, L.; Lin, Z.; Zhao, M. Inhibitory effect of triptolide on glioblastoma multiforme in vitro. J. Int. Med. Res. 2007, 35, 490–496. [Google Scholar]
- Chairungsrilerd, N.; Furukawa, K.; Tadano, T.; Kisara, K.; Ohizumi, Y. Effect of gamma-mangostin through the inhibition of 5-hydroxy-tryptamine 2A receptors in 5-fluoro-alpha-methyltryptamine-induced head-twitch responses of mice. Br. J. Pharmacol. 1998, 123, 855–862. [Google Scholar] [CrossRef]
- Kakiuchi, N.; Hattori, M.; Nishizawa, M.; Yamagishi, T.; Okuda, T.; Namba, T. Studies on dental caries prevention by traditional medicines. VIII. Inhibitory effect of various tannins on glucan synthesis by glucosyltransferase from Streptococcus mutans. Chem. Pharm. Bull. (Tokyo) 1986, 34, 720–725. [Google Scholar] [CrossRef]
- Nakatani, K.; Yamakuni, T.; Kondo, N.; Arakawa, T.; Oosawa, K.; Shimura, S.; Inoue, H.; Ohizumi, Y. gamma-Mangostin inhibits inhibitor-kappaB kinase activity and decreases lipopolysaccharide induced cyclooxygenase-2 gene expression in C6 rat glioma cells. Mol. Pharmacol. 2004, 66, 667–674. [Google Scholar] [CrossRef]
- Yamakuni, T.; Aoki, K.; Nakatani, K.; Kondo, N.; Oku, H.; Ishiguro, K.; Ohizumi, Y. Garcinone B reduces prostaglandin E2 release and NF-kappaB-mediated transcription in C6 rat glioma cells. Neurosci. Lett. 2006, 394, 206–210. [Google Scholar] [CrossRef]
- Balunas, M.J.; Su, B.; Brueggemeier, R.W.; Kinghorn, A.D. Xanthones from the botanical dietary supplement mangosteen (Garcinia mangostana) with aromatase inhibitory activity. J. Nat. Prod. 2008, 71, 1161–1166. [Google Scholar] [CrossRef]
- Chen, S.X.; Wan, M.; Loh, B.N. Active constituents against HIV-1 protease from Garcinia mangostana. Planta Med. 1996, 62, 381–382. [Google Scholar] [CrossRef]
- Chairungsrilerd, N.; Furukawa, K.; Ohta, T.; Nozoe, S.; Ohizumi, Y. Histaminergic and serotonergic receptor blocking substances from the medicinal plant Garcinia mangostana. Planta Med. 1996, 62, 471–472. [Google Scholar] [CrossRef]
- Jung, H.A.; Su, B.N.; Keller, W.J.; Mehta, R.G.; Kinghorn, A.D. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J. Agric. Food Chem. 2006, 54, 2077–2082. [Google Scholar] [CrossRef]
- Akao, Y.; Nakagawa, Y.; Iinuma, M.; Nozawa, Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Int. J. Mol. Sci. 2008, 9, 355–370. [Google Scholar] [CrossRef]
- Chow, J.M.; Huang, G.C.; Shen, S.C.; Wu, C.Y.; Lin, C.W.; Chen, Y.C. Differential apoptotic effect of wogonin and nor-wogonin via stimulation of ROS production in human leukemia cells. J. Cell Biochem. 2008, 103, 1394–1404. [Google Scholar] [CrossRef]
- Wong, S.H.; Knight, J.A.; Hopfer, S.M.; Zaharia, O.; Leach, C.N., Jr; Sunderman, F.W., Jr. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde thiobarbituric acid adduct. Clin. Chem. 1987, 33, 214–220. [Google Scholar]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Chen, Y.C.; Shen, S.C.; Lee, W.R.; Lin, H.Y.; Ko, C.H.; Shih, C.M.; Yang, L.L. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch. Toxicol. 2002, 76, 351–359. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Chu, Y.F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef]
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef]
- Temple, N.J.; Gladwin, K.K. Fruit, vegetables, and the prevention of cancer: Research challenges. Nutrition 2003, 19, 467–470. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, H.-F.; Huang, W.-T.; Chen, H.-J.; Yang, L.-L. Apoptotic Effects of γ-Mangostin from the Fruit Hull of Garcinia mangostana on Human Malignant Glioma Cells. Molecules 2010, 15, 8953-8966. https://doi.org/10.3390/molecules15128953
Chang H-F, Huang W-T, Chen H-J, Yang L-L. Apoptotic Effects of γ-Mangostin from the Fruit Hull of Garcinia mangostana on Human Malignant Glioma Cells. Molecules. 2010; 15(12):8953-8966. https://doi.org/10.3390/molecules15128953
Chicago/Turabian StyleChang, Hui-Fang, Wen-Tsung Huang, Hui-Ju Chen, and Ling-Ling Yang. 2010. "Apoptotic Effects of γ-Mangostin from the Fruit Hull of Garcinia mangostana on Human Malignant Glioma Cells" Molecules 15, no. 12: 8953-8966. https://doi.org/10.3390/molecules15128953




