Evaluation of the Bioactivity of Novel Spiroisoxazoline TypeCompounds against Normal and Cancer Cell Lines
Abstract
:1. Introduction

2. Results and Discussion
2.1. Effects of novel pyrrolidine type compounds on normal and cancer cell lines

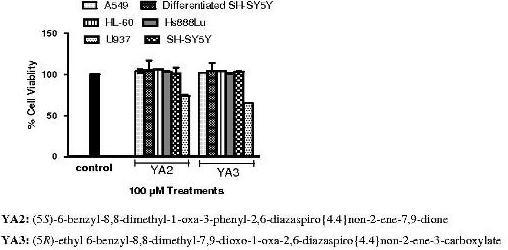
2.2. The cytotoxicity effects of novel spiroisoxazoline type compounds on normal and cancer cell lines


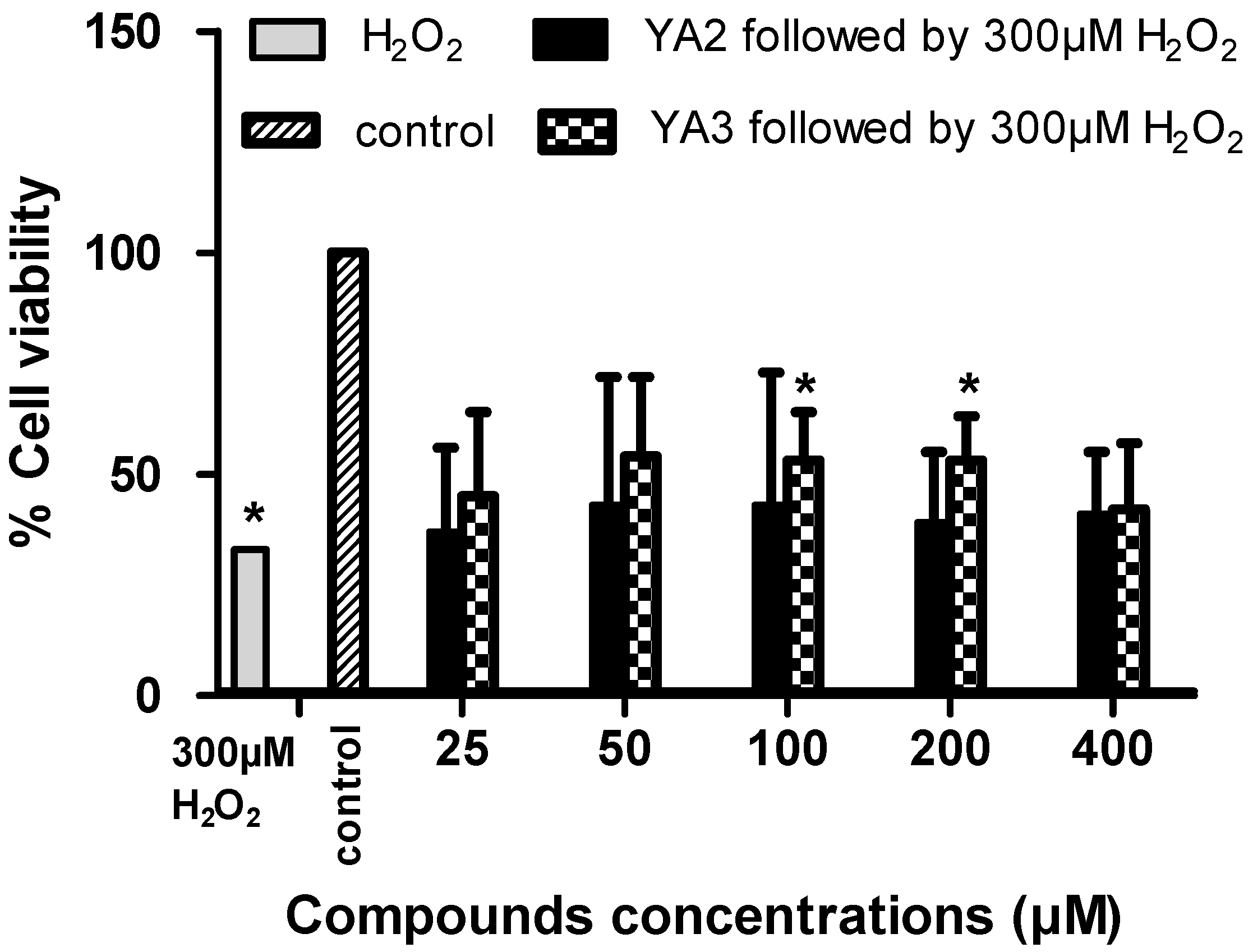
2.3. The neurotoxicity and neuroprotection effects of novel spiroisoxazoline type compounds
2.4. Effects of spiroisoxazoline type compounds on cell cycle and apoptosis levels


3. Experimental
3.1. Chemical Compounds
3.2. Cell lines and culture conditions
3.3. Assay for cytotoxic activity
3.4. Neurotoxicity and neuroprotection activities
3.5. Cell cycle phase distribution
3.6. Apoptosis assay
3.7. Statistical analysis
4. Conclusions
Acknowledgements
References
- Page, P.C.B.; Hamzah, A.S.; Leach, D.C.; Allin, S.M.; Andrews, D.M.; Rassias, G.A. Short and versatile route to a key intermediate for lactacystin synthesis. Org. Lett. 2003, 5, 353–355. [Google Scholar] [CrossRef]
- Page, P.C.B.; Leach, D.C.; Hayman, C.M.; Hamzah, A.S.; Allin, S.M.; McKee, V. A short preparation of an advanced intermediate for lactacystin synthesis: The complete carbon skeleton of clasto-lactacystin dihydroxyacid. Synlett 2003, 2003, 1025–1027. [Google Scholar]
- Bathich, Y.; Mohammat, M.F.; Hamzah, A.S.; Goh, J.H.; Fun, H.-K. (5R)-Ethyl 6-benzyl-8,8-dimethyl-7,9-dioxo-1-oxa-2,6-diazaspiro{4.4}non-2-ene-3-carboxylate. Acta Crystallogr. E. 2009, E65, o2888–o2889. [Google Scholar]
- Mohammat, M.F.; Shaameri, Z.; Hamzah, A.S. Synthesis of 2,3-dioxo-5-(substituted)arylpyrroles and their 2-Oxo-5-aryl-3-hydrazone pyrrolidine derivatives. Molecules 2009, 14, 250–256. [Google Scholar] [CrossRef]
- Kang, Y.K.; Shin, K.J.; Yoo, K.H.; Seo, K.J.; Hong, C.Y.; Lee, C.-S.; Park, S.Y.; Kim, D.J.; Park, S.W. Synthesis and antibacterial activity of new carbapenems containing isoxazole moiety. Bioorg. Med. Chem. Lett. 2000, 10, 95–99. [Google Scholar] [CrossRef]
- Li, X.-F.; Feng, Y.-Q.; Gao, B.; Li, N. 3'''-(2,6-Dichlorophenyl)-1'-methyl-4',4''' diphenyl-4''',5'''-dihydro-indole-3-spiro pyrrolidine-3'-spiro-1''-cyclopentane-3'' spiro-5'''-[1,2]-oxazole-2(3H),2''-dione. Acta Crystallogr., Sect. E: Struct. Rep. Online 2003, E59, o1659–o1660. [Google Scholar]
- Shorvon, S. Pyrrolidone derivatives. Lancet 2001, 358, 1885–1892. [Google Scholar] [CrossRef]
- Fattorusso, E.; Minale, L.; Sodano, G.; Moody, K.; Thomson, R.H. Aerothionin a tetrabromo-compound from Aplysina aerophoba and Verongia thiona. Chem. Commun. 1970, 752–753. [Google Scholar]
- Moody, K.; Thomson, R.H.; Fattorusso, E.; Minale, L.; Sodano, G. Aerothionin and homoaerothionin: two tetrabromo spirohexadienylisoxazoles from Verongia sponges. J. Chem. Soc. Perkin. Trans. 1. 1972, 18–24. [Google Scholar]
- McMillan, J.A.; Paul, I.C.; Goo, Y.M.; Rinehart, J.K.L.; Krueger, W.C.; Pschigoda, L.M. An x-ray study of aerothionin from aplysina fistularis (pallas). Tetrahedron Lett. 1981, 22, 39–42. [Google Scholar] [CrossRef]
- Saeki, B.M.; Granato, A.C.; Berlinck, R.G.S.; Magalhaes, A.; Schefer, A.B.; Ferreira, A.G.; Pinheiro, U.S.; Hajdu, E. Two unprecedented ibromotyrosine-derived alkaloids from the Brazilian endemic marine sponge aplysina caissara. J. Nat. Prod. 2002, 65, 796–799. [Google Scholar] [CrossRef]
- Encarnacion, R.D.; Sandoval, E.; Malmstrom, J.; Christophersen, C. Calafianin, a bromotyrosine derivative from the marine sponge Aplysina gerardogreeni. J. Nat. Prod. 2000, 63, 874–875. [Google Scholar] [CrossRef]
- Ogamino, T.; Obata, R.; Tomoda, H.; Nishiyama, S. Total synthesis, structural revision, and biological evaluation of calafianin, a marine spiroisoxazoline from the sponge, Aplysina gerardogreeni. Bull. Chem. Soc. Japan 2006, 79, 134–139. [Google Scholar] [CrossRef]
- Kobayashi, J.i.; Tsuda, M.; Agemi, K.; Shigemori, H.; Ishibashi, M.; Sasaki, T.; Mikami, Y. Purealidins B and C, new bromotyrosine alkaloids from the okinawan marine sponge psammaplysilla purea. Tetrahedron 1991, 47, 6617–6622. [Google Scholar] [CrossRef]
- Fu, X.; Schmitz, F.J.; Govindan, M.; Abbas, S.A.; Hanson, K.M.; Horton, P.A.; Crews, P.; Laney, M.; Schatzman, R.C. Enzyme inhibitors: new and known polybrominated phenols and diphenyl ethers from four indo-pacific dysidea sponges. J. Nat. Prod. 1995, 58, 1384–1391. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ojika, M.; Kato, S.; Sakagami, Y. Ianthesines, A-D, four novel dibromotyrosine-derived metabolites from a marine sponge. Ianthella sp. Tetrahedron 2000, 56, 5813–5818. [Google Scholar]
- Nicholas, G.M.; Eckman, L.L.; Ray, S.; Hughes, R.O.; Pfefferkorn, J.A.; Barluenga, S.; Nicolaoub, K.C.; Bewleya, C.A. Bromotyrosine-derived natural and synthetic products as inhibitors of mycothiol-S-onjugate amidase. Bioorg. Med. Chem. Lett. 2002, 12, 2487–2490. [Google Scholar] [CrossRef]
- Trujillo-Jimenez, F.; Garcia-Lopez, P.; Garci-Carranca, A.; Abdullaev, F.I. Effect of saffron on the viability of normal and malignant human cells in vitro. Acta Horticult. 2004, 1, 463–469. [Google Scholar]
- Yamashita, M.; Kaneko, M.; Tokuda, H.; Nishimura, K.; Kumeda, Y.; Iida, A. Synthesis and evaluation of bioactive naphthoquinones from the Brazilian medicinal plant, Tabebuia avellanedae. Bioorg. Med. Chem. 2009, 17, 6286–6291. [Google Scholar] [CrossRef]
- Takeo, S.; Miyake, K.; Tanonaka, K.; Takagi, N.; Takagi, K.; Kishimoto, K. Beneficial effect of nebracetam on energy metabolism after microsphere-induced embolism in rat brain. Arch Int Pharmacodyn. Ther. 1996, 331, 232–245. [Google Scholar]
- Ohjimi, H.; Kushiku, K.; Yamada, H.; Kuwahara, T.; Kohno, Y.; Furukawa, T. Increase of acetylcholine release by nebracetam in dog cardiac sympathetic ganglion. J. Pharmacol. Exp. Ther 1994, 268, 396–402. [Google Scholar]
- Nakamura, K.; Shirane, M. Activation of the reticulothalamic cholinergic pathway by the major metabolites of aniracetam. Eur. J. Pharmacol. 1999, 380, 81–89. [Google Scholar] [CrossRef]
- Oyaizu, M.; Narahashi, T. Modulation of the neuronal nicotinic acetylcholine receptor-channel by the nootropic drug nefiracetam. Brain Res. 1999, 822, 72–79. [Google Scholar] [CrossRef]
- Birnstiel, S.; Wülfert, E.; Beck, S. Levetiracetam (UCB L059) affects in vitro models of epilepsy in CA3 pyramidal neurons without altering normal synaptic transmission. Arch. Pharmacol. 1997, 356, 611–618. [Google Scholar] [CrossRef]
- Niespodziany, I.; Klitgaard, H.; Margineaunu, D.-G. Levetiracetam: inhibits high voltage-activated Ca2+ current in CA1 pyramidal neurons of rat hippocampal slices. Neurosci. Lett. 2001, 306, 5–8. [Google Scholar] [CrossRef]
- Black, M.; Wotanis, J.; Schilp, D.; Hanak, S.; Sorensen, S.; Wettstein, J. Effect of AMPA receptor modulators on hippocampal and cortical function. Eur. J. Pharmacol. 2000, 394, 85–90. [Google Scholar] [CrossRef]
- Kolta, A.; Lynch, G.; Ambros Ingerson, J. Effects of aniracetam after LTP induction are suggestive of interactions on the kinetics of the AMPA receptor channel. Brain Res. 1998, 788, 269–286. [Google Scholar] [CrossRef]
- Lu, Y.; Wehner, J. Enhancement of contextual fear-conditioning by putative (+/-)-alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor modulators and N-methyl-D-aspartate (NMDA) receptor antagonists in DBA/2J mice. Brain Res. 1997, 768, 197–207. [Google Scholar] [CrossRef]
- Peuvot, J.; Schanck, A.; Deleers, M.; Brasseur, R. Piracetam-induced changes to membrane physical properties: a combined approach by 31P nuclear magnetic resonance and conformational analysis. Biochem. Pharmacol. 1995, 50, 1129–1134. [Google Scholar] [CrossRef]
- Müller, W.; Koch, S.; Rostock, A.; Bartsch, R. Effects of piracetam on membrance fluidity in the aged mouse, rat and human brain. Biochem. Pharmacol. 1997, 53, 135–140. [Google Scholar]
- Pinteaux, E.; Copin, J.C.; Ledig, M.T. Modulation of oxygen-radical scavenging enzymes by oxidative stress in primary cultures of rat astroglial cells. Dev. Neurosci. 1996, 18, 397–404. [Google Scholar] [CrossRef]
- Dringen, R.; Pawlowski, P.G.; Hirrlinger, J. Peroxide detoxification by brain cells. Neurosci. Res. 2005, 79, 157–165. [Google Scholar] [CrossRef]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar]
- Perckel, T.; Luedke, G. Apoptosis detection by annexin V and active caspase-3 with the Agilent 2100 bioanalyzer Bioanalyzer. Agilent Appl. Note 2001, 5988–4319EN. [Google Scholar]
- Rossi, D.; Gaidano, G. Messengers of cell death: apoptotic signaling in health and disease. Haematologica 2003, 88, 212–218. [Google Scholar]
- Tsuruo, T.; Naito, M.; Tomida, A.; Fujita, N.; Mashima, T.; Sakamoto, H.; Haga, N. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer. Sci. 2003, 94, 15–21. [Google Scholar]
- Debatin, K.M. Apoptosis pathways in cancer and cancer therapy. Cancer. Immunol. Immunother. 2004, 53, 153–197. [Google Scholar] [CrossRef]
- Liu, J.-J.; Zhang, Y.; Guang, W.-B.; Yang, H.-Z.; Lin, D.-J.; Xiao, R.-Z. Ponicidin inhibits monocytic leukemia cell growth by induction of apoptosis. Int. J. Mol. Sci. 2008, 9, 2265–2277. [Google Scholar] [CrossRef]
- Han, M.H.; Yoo, Y.H.; Choi, Y.H. Sanguinarine-induced apoptosis in human leukemia U937 cells via Bcl-2 downregulation and caspase-3 activation. Chemotherapy 2008, 54, 157–165. [Google Scholar] [CrossRef]
- Park, J.-W.; Choi, Y.-J.; Suh, S.-I.; Baek, W.-K.; Suh, M.-H.; Jin, I.-N.; Min, D.S.; Woo, J.-H.; Chang, J.-S.; Passaniti, A.; Lee, Y.H.; Kwon, T.K. Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Carcinogenesis 2001, 22, 1633–1639. [Google Scholar]
- Filomeni, G.; Rotilio, G.; Ciriolo, M.R. Glutathione disulfide induces apoptosis in U937 cells by a redox-mediated p38 MAP kinase pathway. FASEB J. 2003, 17, 64–66. [Google Scholar] [Green Version]
- Qin-Hong, W.; Yi, X. HMBA - induced differentiation of myeloid leukemic cell lines is associated with altered G1 cell cycle regulators and genes. Chin. Med. J. 2004, 117, 453–455. [Google Scholar]
- Moon, D.-O.; Park, C.; Heo, M.-S.; Park, Y.-M.; Choi, Y.H.; Kim, G.-Y. PD98059 triggers G1 arrest and apoptosis in human leukemic U937 cells through downregulation of Akt signal pathway. Int. Immunopharmacol. 2007, 7, 36–45. [Google Scholar] [CrossRef]
- Bertram, C.; Neuhoff, N.v.; Skawran, B.; Steinemann, D.; Schlegelberger, B.; Hass, R. The differentiation/retrodifferentiation program of human U937 leukemia cells is accompanied by changes of VCP/p97. BMC Cell Biol. 2008, 9, 1–16. [Google Scholar] [CrossRef]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Ceña, V.; Gallego, C.; Comella, J.X. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar]
- Singh, J.; Kaur, G. Transcriptional regulation of polysialylated neural cell adhesion molecule expression by NMDA receptor activation in retinoic acid-differentiated SH-SY5Y neuroblastoma cultures. Brain Res. 2007, 1154, 8–21. [Google Scholar] [CrossRef]
- Kalejta, R.F.; Shenk, T.; Beavis, A.J. Use of a membrane-localized green fluorescent protein allows simultaneous analysis of transfected cells and cell cycle analysis by flow cytometry. Cytometry 1997, 29, 286–291. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Najim, N.; Bathich, Y.; Zain, M.M.; Hamzah, A.S.; Shaameri, Z. Evaluation of the Bioactivity of Novel Spiroisoxazoline TypeCompounds against Normal and Cancer Cell Lines. Molecules 2010, 15, 9340-9353. https://doi.org/10.3390/molecules15129340
Najim N, Bathich Y, Zain MM, Hamzah AS, Shaameri Z. Evaluation of the Bioactivity of Novel Spiroisoxazoline TypeCompounds against Normal and Cancer Cell Lines. Molecules. 2010; 15(12):9340-9353. https://doi.org/10.3390/molecules15129340
Chicago/Turabian StyleNajim, Nigar, Yaser Bathich, Mazatulikhma Mat Zain, Ahmad Sazali Hamzah, and Zurina Shaameri. 2010. "Evaluation of the Bioactivity of Novel Spiroisoxazoline TypeCompounds against Normal and Cancer Cell Lines" Molecules 15, no. 12: 9340-9353. https://doi.org/10.3390/molecules15129340