The Influence of Mixed Activators on Ethylene Polymerization and Ethylene/1-Hexene Copolymerization with Silica-Supported Ziegler-Natta Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst characterization

2.2. Ethylene homo-polymerization

| Run number | Activatora | Al/Ti | Activityb (kg PE/ g cat/ h/ atm) | ||
|---|---|---|---|---|---|
| TEA (mol%) | DEAC (mol%) | TnHA (mol%) | |||
| 1 | 100 | - | - | 100 | 0.53 |
| 2 | 100 | - | - | 100 | 0.55 |
| 3 | 100 | - | - | 300 | 0.83 |
| 4 | - | 100 | - | 100 | 0.32 |
| 5 | - | 100 | - | 100 | 0.30 |
| 6 | - | 100 | - | 300 | 0.48 |
| 7 | - | - | 100 | 100 | 0.78 |
| 8 | - | - | 100 | 100 | 0.76 |
| 9 | - | - | 100 | 300 | 1.10 |
| 10 | 50 | 50 | - | 100 | 1.79 |
| 11 | 50 | 50 | - | 300 | 2.25 |
| 12 | 50 | - | 50 | 100 | 1.25 |
| 13 | 50 | - | 50 | 300 | 1.71 |
| 14 | 33 | 33 | 33 | 100 | 2.23 |
| 15 | 33 | 33 | 33 | 300 | 2.86 |


2.3. Ethylene/1-hexene copolymerization
| Run number | Activatora used | Activity (kg polymer/g cat/ h/ atm) | 1-hexene insertion (mol%)b | ||
|---|---|---|---|---|---|
| TEA (mol%) | DEAC (mol%) | TnHA (mol%) | |||
| 16 | 100 | - | - | 2.53 | 1.27 |
| 17 | - | 100 | - | 1.50 | 0.49 |
| 18 | - | - | 100 | 1.91 | 1.90 |
| 19 | 50 | 50 | - | 3.22 | 0.70 |
| 20 | 50 | - | 50 | 4.15 | 1.26 |
| 21 | - | 50 | 50 | 2.75 | 0.60 |
| 22 | 33 | 33 | 33 | 5.69 | 1.10 |


2.4. Polymer characterization
2.4.1. Gel permeation chromatography (GPC) analysis
| Run | Monomer | Activator | Mn (kg/ mol) | Mw (kg/ mol) | Mw/Mna | Tmb (°C) | dc (g/mL) | Crystallinity (%) | |
|---|---|---|---|---|---|---|---|---|---|
| DSCd | X-raye | ||||||||
| 3 | Ethylene | TEA | 74 | 299 | 4.0 | 136.5 | 0.955 | 66.0 | 71.3 |
| 6 | DEAC | 51 | 180 | 3.5 | 136.3 | 0.957 | 67.7 | - | |
| 9 | TnHA | 103 | 474 | 4.6 | 135.9 | 0.956 | 66.3 | - | |
| 11 | TEA+ DEAC | 31 | 175 | 5.6 | 136.3 | 0.957 | 67.7 | - | |
| 13 | TEA+ TnHA | 86 | 344 | 4.0 | 135.9 | 0.957 | 67.7 | - | |
| 15 | TEA+ DEAC+ TnHA | 55 | 283 | 5.2 | 136.3 | 0.957 | 67.6 | - | |
| 16 | Ethylene/ 1-hexene | TEA | 165 | 295 | 3.8 | 120.7 | 0.926 | 41.0 | 46.2 |
| 17 | DEAC | 56 | 364 | 6.5 | 124.4 | 0.943 | 55.5 | 60.1 | |
| 18 | TnHA | 79 | 336 | 4.2 | 97.5 | 0.914 | 30.7 | 35.4 | |
| 19 | TEA+ DEAC | 77 | 294 | 3.8 | 122.5 | 0.941 | 54.0 | 59.1 | |
| 20 | TEA+ TnHA | 56 | 255 | 4.5 | 111.6 | 0.918 | 34.1 | 38.2 | |
| 21 | TnHA+ DEAC | 54 | 209 | 3.8 | 123.7 | 0.943 | 55.3 | 60.3 | |
| 22 | TEA+ DEAC+ TnHA | 48 | 272 | 5.6 | 119.5 | 0.923 | 38.4 | 42.2 | |
2.4.2. X-ray diffraction (XRD) and thermal properties
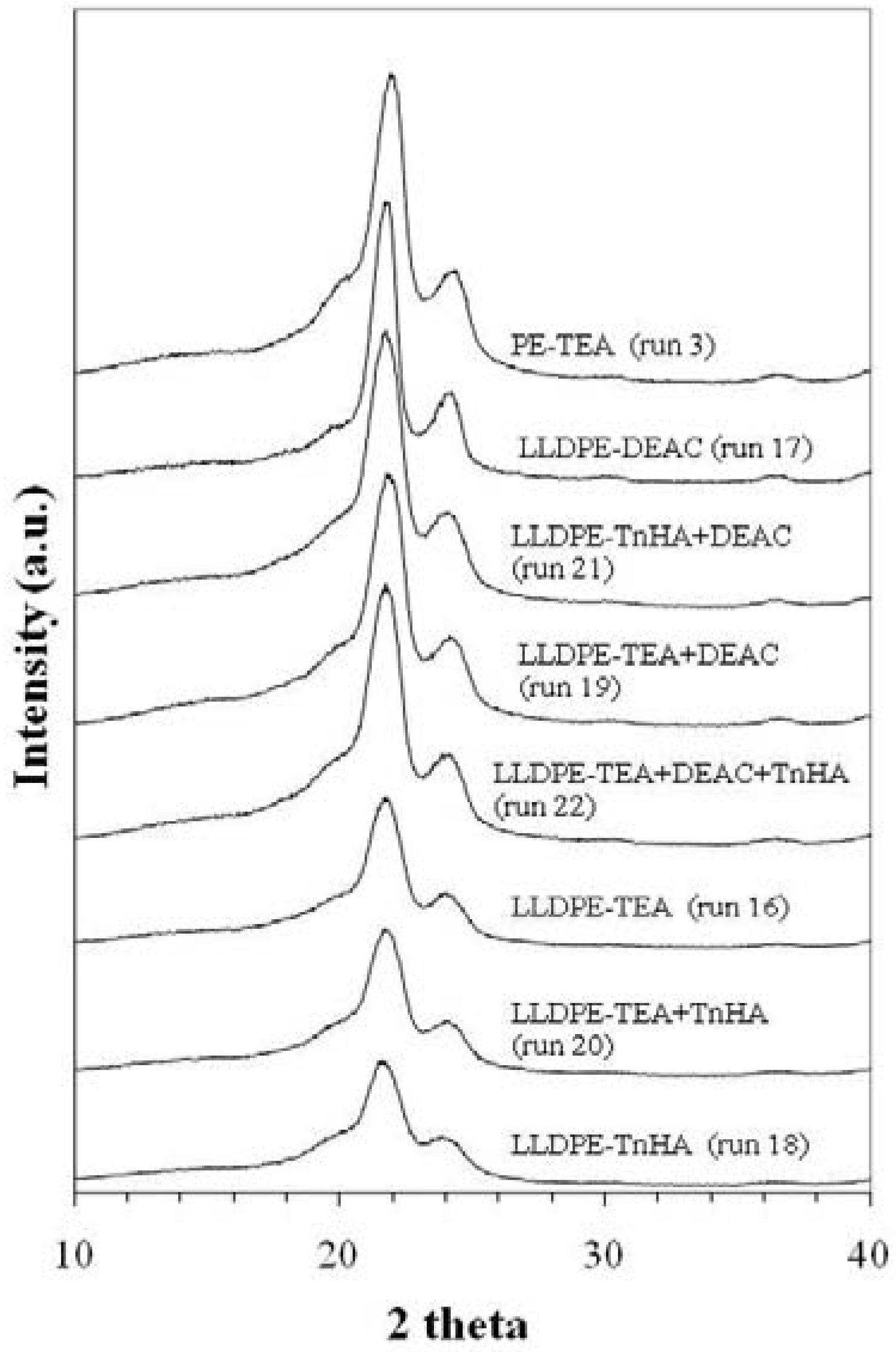
| Run | Activator | [HHH] | [EHH] | [EHE] | [EEE] | [HEH] | [HEE] | %E | %H |
|---|---|---|---|---|---|---|---|---|---|
| 16 | TEA | 0.0 | 0.7 | 0.6 | 97.0 | 0.2 | 1.2 | 98.73 | 1.27 |
| 17 | DEAC | 0.0 | 0.2 | 0.3 | 98.8 | 0.1 | 0.6 | 99.51 | 0.49 |
| 18 | TnHA | 0.0 | 1.2 | 0.7 | 96.3 | 0.4 | 1.4 | 98.10 | 1.90 |
| 19 | TEA+DEAC | 0.0 | 0.2 | 0.5 | 98.2 | 0.1 | 1.0 | 99.30 | 0.70 |
| 20 | TEA+TnHA | 0.0 | 0.7 | 0.6 | 97.2 | 0.2 | 1.2 | 98.74 | 1.26 |
| 21 | TnHA+DEAC | 0.0 | 0.2 | 0.4 | 98.4 | 0.2 | 0.8 | 99.40 | 0.60 |
| 22 | TEA+DEAC+TnHA | 0.0 | 0.4 | 0.7 | 97.4 | 0.1 | 1.4 | 98.89 | 1.11 |
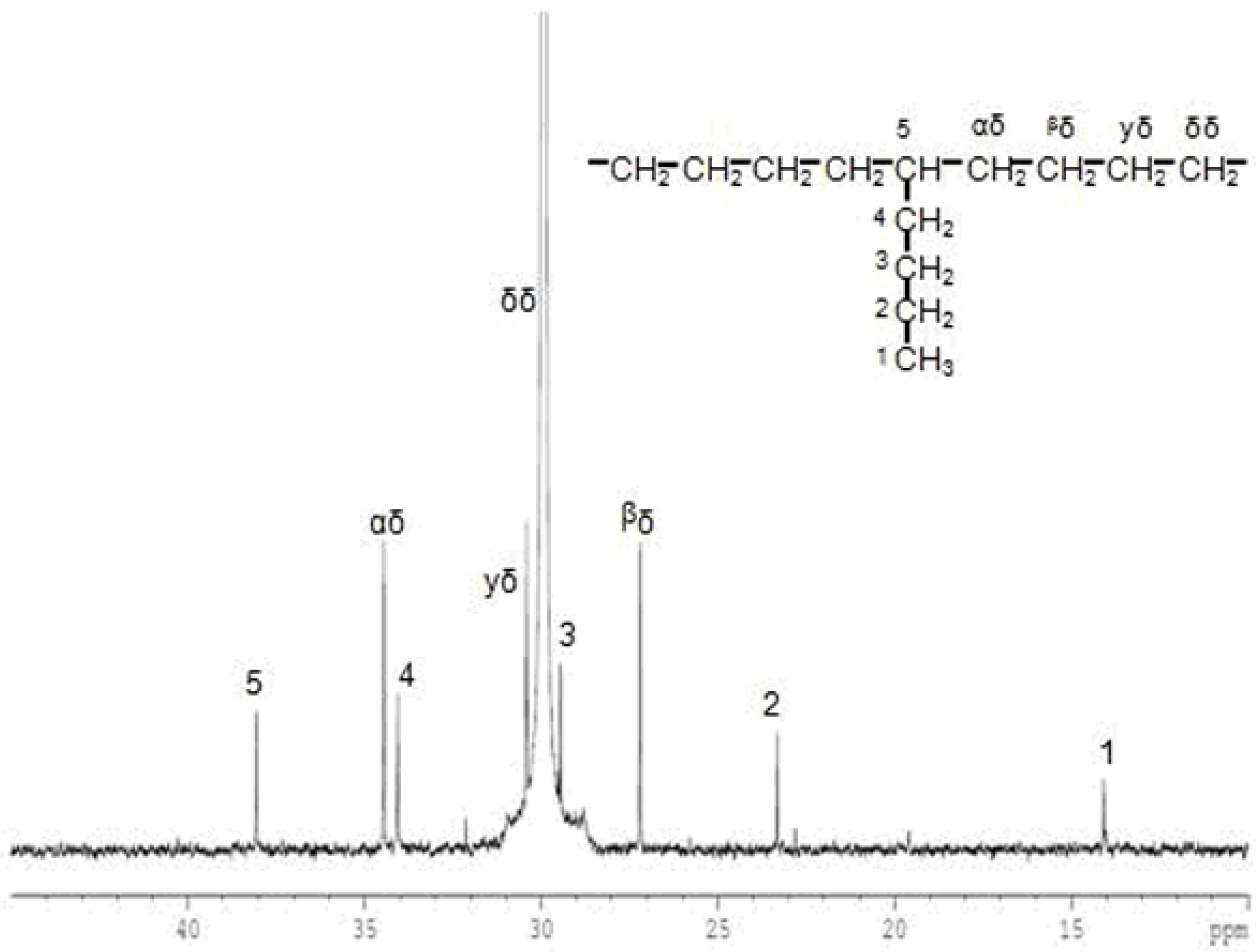
3. Experimental
3.1. Chemicals
3.2. Catalyst preparation
3.3. Polymerization reaction
3.4. Polymer characterization

4. Conclusions
Acknowledgements
References and Notes
- Quijada, R.; Guevara, J.L.; Galland, G.B.; Rabagliati, F.M.; Lopez-Majada, J.M. Synthesis and properties coming from the copolymerization of propene with α-olefins using different metallocene catalysts. Polymer 2005, 46, 1567–1574. [Google Scholar]
- Kawahara, N.; Kojoh, S.; Toda, Y.; Mizuno, A.; Kashiwa, N. The detailed analysis of the vinylidene structure of metallocene-catalyzed polypropylene. Polymer 2004, 45, 355–357. [Google Scholar]
- Cruz, V.; Ramos, J.; Munˇoz-Escalona, A.; Lafuente, P.; Penˇa, B.; Martinez, S. 3D-QSAR analysis of metallocene-based catalysts used in ethylene polymerization. Polymer 2004, 45, 2061–2072. [Google Scholar]
- Lee, H.W.; Chung, J.S.; Choi, K.Y. Physical transitions and nascent morphology of syndiotactic polystyrene in slurry polymerization with embedded Cp*Ti(OMe)3/methyl aluminoxane catalyst. Polymer 2005, 46, 5032–5039. [Google Scholar] [CrossRef]
- Nitta, K.H.; Shin, Y.W.; Hashiguchi, H.; Tanimoto, S.; Terano, T. Morphology and mechanical properties in the binary blends of isotactic polypropylene and novel propylene-co-olefin random copolymers with isotactic propylene sequence 1. Ethylene-propylene copolymers. Polymer 2005, 46, 965–975. [Google Scholar]
- Wooster, T.J.; Abrol, S.; Macfarlane, D.R. Cyanate ester polymerization catalysis by layered-silicates. Polymer 2004, 45, 7845–7852. [Google Scholar] [CrossRef]
- Chen, S.; Hua, Z.J.; Fang, Z.; Qi, G.R. Copolymerization of carbon dioxide and propylene oxide with highly effective zinc hexacyanocobaltate(III)-based coordination catalyst. Polymer 2004, 45, 6519–6524. [Google Scholar] [CrossRef]
- Bazzini, C.; Giarrusso, A.; Porri, L.; Pirozzi, B.; Napolitano, R. Synthesis and characterization of syndiotactic 3,4-polyisoprene prepared with diethylbis(2,2'-bipyridine)iron-MAO. Polymer 2004, 45, 2871–2875. [Google Scholar]
- Liu, J.Y.; Zheng, Y.; Li, Y.S. Polymerization of methyl methacrylate by iron(II) pyridinebisimine complexes. Polymer 2004, 45, 2297–2301. [Google Scholar]
- Gao, M.Z.; Liu, H.T.; Wang, J.; Li, C.X.; Ma, J.; Wei, G.S. Novel MgCl2-supported catalyst containing diol dibenzoate donor for propylene polymerization. Polymer 2004, 45, 2175–2180. [Google Scholar]
- Kawahara, N.; Kojoh, S.I.; Toda, Y.; Mizuno, A.; Kashiwa, N. The detailed analysis of the vinylidene structure of metallocene-catalyzed polypropylene. Polymer 2004, 45, 355–357. [Google Scholar] [CrossRef]
- Lee, T.S.; Kim, J.W.; Bae, J.Y. Palladium-catalyzed selective dehalogenative homocoupling polymerization of AB2-type dihaloaryl sulfonate monomers. Polymer 2004, 45, 5065–5076. [Google Scholar]
- Galland, G.B.; Quijada, R.; Rojas, R.; Bazan, G.; Zachary Komon, J.A. NMR Study of Branched Polyethylenes Obtained with Combined Fe and Zr Catalysts. Macromolecules 2002, 35, 339–345. [Google Scholar] [CrossRef]
- Mo, Z.S.; Zhang, H.F. The degree of crystallinity in polymers by wide-angle x-ray diffraction (WAXD). Macromol. Chem. Phys. 1995, C35, 555–580. [Google Scholar]
- Madri, S.; Xuejing, Z.; RoBert, B.J.; JOHN, C.C.; COR, E.K. Effect of 1-Hexene Comonomer on Polyethylene Particle Growth and Copolymer Chemical Composition Distribution. J. Polym. Sci.: Part A: Polym. Chem. 2006, 44, 2883–2890. [Google Scholar] [CrossRef]
- Yong, P.C.; Zhi, F. Ethylene/1-hexene copolymerization with TiCl4/MgCl2/AlCl3 catalyst in the presence of hydrogen. Eur. Polym. J. 2006, 42, 2441–2449. [Google Scholar] [CrossRef]
- Nooijen, G.A.H. On the importance of diffusion of cocatalyst molecules through heterogeneous ziegler/natta catalysts. Eur. Polym. J. 1994, 30, 11–15. [Google Scholar] [CrossRef]
- Lynch, T.D.; Jejelowo, M.O.; Wanke, S.E. The influence of aluminum alkyls on the polymerization of ethylene with silica/magnesium chloride-supported titanium tetrachloride catalysts. Can. J. Chem. Eng. 1991, 69, 657–664. [Google Scholar] [CrossRef]
- Siokou, A.; Ntais, S. Towards the preparation of realistic model Ziegler-Natta catalysts: XPS study of the MgCl2/TiCl4 interaction with flat SiO2/Si(1 0 0). Surf. Sci. 2003, 540, 379–388. [Google Scholar] [CrossRef]
- Dong, Q.; Fu, Z.; Xu, J.; Fan, Z. Strong influences of cocatalyst on ethylene/propylene copolymerization with a MgCl2/SiO2/TiCl4/diester type Ziegler-Natta catalyst. Eur. Polym. J. 2007, 43, 3442–3451. [Google Scholar] [CrossRef]
- Lynch, D.T.; Wanke, S.E. Reactor Design and Operation for Gas-Phase Ethylene Polymerization Using Ziegler-Natta Catalysts. Can. J. Chem. Eng. 1991, 69, 332–339. [Google Scholar] [CrossRef]
- Hammawa, H.; Mannan, T.M.; Lynch, D.T.; Wanke, S.E. Effects of aluminum alkyls on ethylene/1-hexene polymerization with supported metallocene/MAO catalysts in the gas phase. J. Appl. Polym. Sci. 2004, 92, 3549–3560. [Google Scholar] [CrossRef]
- Fukuda, K.; Liu, B.; Nakatani, H.; Nishiyama, I.; Yamahiro, M.; Terano, M. Significant variation of molecular weight distribution (MWD) of polyethylene induced by different alkyl-Al co-catalysts using a novel surface functionalized SiO2-supported Ziegler-Natta catalyst. Catal. Commun. 2003, 4, 657–662. [Google Scholar] [CrossRef]
- Haward, R.N.; Roper, A.N.; Fletcher, K.L. Highly active catalysts for ethylene polymerization by the reduction of TiCl4 with organomagnesium compounds. Polymer 1973, 14, 365–372. [Google Scholar] [CrossRef]
- Gardner, K.; Parsons, I.W.; Haward, R.N. Polymerization of propene with organomagnesium-reduced titanium (IV) chloride-based catalyst. J. Polym. Sci.: Polym. Chem. 1978, 16, 1683–1696. [Google Scholar]
- Kashiwa, N. Super active catalyst for olefin polymerization. Polymer 1980, 12, 603–608. [Google Scholar] [CrossRef]
- Licchelli, J.A.; Haward, R.N.; Parsons, I.W.; Caunt, A.D. Polymerization catalysts for propene from the reduction of titanium tetrachloride with halogen-free magnesium alkyls. Polymer 1981, 22, 667–672. [Google Scholar] [CrossRef]
- Machon, J.P.; Hermant, R.; Houzeaux, J.P. Study of the catalytic activity of violet titanium trichloride in the high-temperature polymerization of ethylene. J. Polym. Sci. Symp. Ser. 1975, 52, 107–117. [Google Scholar]
- Munoz, E.A.; Hernandez, J.G.; Gallardo, J.A.; Keii, T.; Soga, K. Design of supported Ziegler-Natta catalysts using silica as carrier. Stud. Surf. Sci. Catal. 1986, 25, 123–125. [Google Scholar] [CrossRef]
- Munoz, E.A.; Garcia, H.; Albornoz, A. Homo- and copolymerization of ethylene with highly active catalysts based on titanium tetrachloride and Grignard compounds. J. Appl. Polym. Sci. 1987, 34, 977–988. [Google Scholar] [CrossRef]
- Zakharov, V.A.; Bukatov, G.D.; Ermakov, Y. The Mechanism of the Catalytic Polymerisation of Olefins Based on the Number of Active Centres and the Rate Constants for Individual Stages. Russ. Chem. Rev. 1980, 49, 1097–1111. [Google Scholar] [CrossRef]
- Mori, H.; Ohnishi, K.; Terano, M. Ethene polymerization with modified-polypropene-supported highly stable Ziegler catalyst. Macromol. Rapid Commun. 1996, 17, 25–29. [Google Scholar] [CrossRef]
- Zacca, J.J.; Debling, J.A.; Ray, W.H. Reactor residence time distribution effects on the multistage polymerization of olefins - I. Basic principles and illustrative examples, polypropylene. Chem. Eng. Sci. 1996, 51, 4859–4886. [Google Scholar] [CrossRef]
- Kim, I.; Chung, M.C.; Choi, H.K.; Kim, J.H.; Woo, S.I. (1990) Homo- and Co-polymerization of Ethylene with the Highly Active TiCl4/THF/MgCl2 Catalyst. Catal. Olefin Polym. 1990, 323. [Google Scholar]
- Kim, I.; Kim, J.H.; Woo, S.I. Kinetic Study of Ethylene Polymerization by Highly Active Silica Supported TiCl2 MgCl2 Catalyst. J. Appl. Polym. Sci. 1990, 39, 837–854. [Google Scholar] [CrossRef]
- Hatada, K.; Yuki, H. Alkyl interchange in the mixture of triethyl aluminum and diethylaluminum chloride. Tetrahedron Lett. 1967, 51, 5227–5231. [Google Scholar] [CrossRef]
- Calabro, D.C.; Lo, F.Y. A comparison of the reaction kinetics for the homo- and copolymerization of ethylene and hexene with a heterogeneous Ziegler catalys. In Transition Metal Cata lyzed Polymerizations: Ziegler–Natta and Methathesis Polymerization; Quirk, R.P., Ed.; Cambridge University Press: New York, NY, USA, 1988; pp. 729–739. [Google Scholar]
- Chien, J.C.W.; Nozaki, T. Ethylene-hexene copolymerization by heterogeneous and homogeneous Ziegler-Natta catalysts and the "comonomer" effect. J. Polym. Sci. 1993, 31, 227–237. [Google Scholar]
- Randall, J.C. A review of high-resolution liquid carbon-13 nuclear magnetic resonance characterizations of ethylene-based polymers. AJMS-REV. Macromol. Chem. Phys. 1989, C29, 201–317. [Google Scholar] [CrossRef]
- Zakharov, V.A.; Bukatov, G.D.; Yermakov, Y.I. On the mechanism of olefin polymerization by Ziegler-Natta catalysts. Adv. Polym. Sci. 1983, 51, 61–100. [Google Scholar] [CrossRef]
- Carlini, C.; Alessio, A.D.; Giaiacopi, S.; Po, R.; Pracella, M.; Galletti, A.M.R.; Sbrana, G. Linear low-density polyethylenes by co-polymerization of ethylene with 1-hexene in the presence of titanium precursors and organoaluminium co-catalysts. Polymer. 2007, 48, 1185–1192. [Google Scholar] [CrossRef]
- Wunderlich, B.; Czornyj, G. A study of equilibrium melting of polyethylene. Macromolecules 1977, 10, 906–913. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds (PEs and LLDPEs) are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Senso, N.; Khaubunsongserm, S.; Jongsomjit, B.; Praserthdam, P. The Influence of Mixed Activators on Ethylene Polymerization and Ethylene/1-Hexene Copolymerization with Silica-Supported Ziegler-Natta Catalyst. Molecules 2010, 15, 9323-9339. https://doi.org/10.3390/molecules15129323
Senso N, Khaubunsongserm S, Jongsomjit B, Praserthdam P. The Influence of Mixed Activators on Ethylene Polymerization and Ethylene/1-Hexene Copolymerization with Silica-Supported Ziegler-Natta Catalyst. Molecules. 2010; 15(12):9323-9339. https://doi.org/10.3390/molecules15129323
Chicago/Turabian StyleSenso, Nichapat, Supaporn Khaubunsongserm, Bunjerd Jongsomjit, and Piyasan Praserthdam. 2010. "The Influence of Mixed Activators on Ethylene Polymerization and Ethylene/1-Hexene Copolymerization with Silica-Supported Ziegler-Natta Catalyst" Molecules 15, no. 12: 9323-9339. https://doi.org/10.3390/molecules15129323




