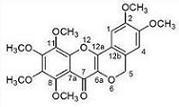
Flavonoids from the Stems of Croton caudatus Geisel. var. tomentosus Hook
Abstract
:1. Introduction
2. Results and Discussion
| Position | δH J (Hz) | δC | HMBC |
|---|---|---|---|
| 1 | 7.02 (1H, s) | 105.5 | C-4a,C-12a,C-3 |
| 2 | 150.4 | ||
| 3 | 152.8 | ||
| 4 | 7.35 (1H, s) | 109.2 | C-5,C-12b,C-2 |
| 5 | 5.14 (2H, s) | 68.0 | C-4,C-12b,C-4a,C-6a |
| 7 | 169.4 | ||
| 8 | 149.0 | ||
| 9 | 144.5 | ||
| 10 | 151.6 | ||
| 11 | 138.7 | ||
| 4a | 126.2 | ||
| 6a | 136.8 | ||
| 7a | 114.6 | ||
| 11a | 146.5 | ||
| 12a | 146.9 | ||
| 12b | 118.1 | ||
| MeO-2 | 3.94 (3H, s) | 56.2 | C-2 |
| MeO-3 | 3.91 (3H, s) | 56.2 | C-3 |
| MeO-8 | 3.85 (3H, s) | 61.9 | C-8 |
| MeO-9 | 3.88 (3H, s) | 61.6 | C-9 |
| MeO-10 | 4.08 (3H, s) | 61.6 | C-10 |
| MeO-11 | 4.08 (3H, s) | 61.6 | C-11 |

3. Experimental Section
3.1. General
3.2. Plant material
3.3. Extraction and isolation
4. Conclusions
Acknowledgements
References and Notes
- Qiu, H.X. Florae Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1996; Volume 44, p. 125. [Google Scholar]
- Morales, A.; Pérez, P.; Mendoza, R.; Compagnone, R.; Suarez, A.I.; Arvelo, F.; Ramírez, J.L.; Galindo-Castro, I. Cytotoxic and proapoptotic activity of ent-16beta-17alpha-dihydroxykaurane on human mammary carcinoma cell line MCF-7. Cancer Lett. 2005, 218, 109–116. [Google Scholar] [CrossRef]
- Kuo, P.C.; Shen, Y.C.; Yang, M.L.; Wang, S.H.; Thang, T.D.; Dung, N.X.; Chiang, P.C.; Lee, K.H.; Lee, E.J.; Wu, T.S. Crotonkinins A and B and related diterpenoids from Croton tonkinensis as anti-inflammatory and antitumor agents. J. Nat. Prod. 2007, 70, 1906–1909. [Google Scholar] [CrossRef]
- Kitazawa, E.; Sato, A.; Takahashi, S.; Kuwano, H.; Ogiso, A. Novel diterpenelactones with anti-peptic ulcer activity from Croton sublyratus. Chem. Pharm. Bull. 1980, 28, 227–234. [Google Scholar] [CrossRef]
- Guerrero, M.F.; Puebla, P.; Carrón, R.; Martín, M.L.; San Román, L. Quercetin 3,7-dimethyl ether: A vasorelaxant flavonoid isolated from Croton schiedeanus Schlecht. J. Pharm. Pharmacol. 2002, 54, 1373–1378. [Google Scholar]
- González-Vázquez, R.; Diaz, B.K.; Aguilar, M.I.; Diego, N.; Lotina-Hennsen, B. Pachypodol from Croton ciliatoglanduliferus Ort. as Water-Splitting Enzyme Inhibitor on Thylakoids. J. Agric. Food Chem. 2006, 54, 1217–1221. [Google Scholar] [CrossRef]
- Palmeira, S.F., Jr.; Conserva, L.M.; Silveira, E.R. Two clerodane diterpenes and flavonoids from Croton brasiliensis. J. Braz. Chem. Soc. 2005, 16, 1420–1424. [Google Scholar] [CrossRef]
- Jiangsu New Medical College, Dictionary of Traditional Chinese Medicine; Shanghai Science and Technology Press: Shanghai, China, 1975; p. 447.
- Pharmacopoeia Commission of People’s Republic of China, Pharmacopoeia of the Peoples Republic of China; Chemical Industry Press: Beijing, China, 2005; Volume I, p. 306.
- Wang, Y.; Zou, Z.M. Sesquiterpenes from the Stems of Croton caudatus Geisel. var. tomentosus Hook. Chin. J. Nat. Med. 2008, 6, 339–341. [Google Scholar]
- Li, S.M.; Lo, C.Y.; Ho, C.T. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. J. Agric. Food Chem. 2006, 54, 4176–4185. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Huang, X.; Tu, Y.; Ni, K. Identification of polymethoxylated flavones from green tangerine peel (Pericarpium Citri Reticulatae Viride) by chromatographic and spectroscopic techniques. J. Pharm. Biomed. Anal. 2007, 44, 63–69. [Google Scholar] [CrossRef]
- Horie, T.; Ohtsuru, Y.; Shibata, K.; Yamashita, K.; Tsukayama, M.; Kawamura, Y. 13C NMR spectral assignment of the A-ring of polyoxygenated flavones. Phytochemistry 1998, 47, 865–874. [Google Scholar] [CrossRef]
- Budzianowski, J. Kaempferol glycosides from Hosta ventricosa. Phytochemistry 1990, 29, 3643–3647. [Google Scholar] [CrossRef]
- Budzianowski, J.; Skrzypczak, L. Phenylpropanoid esters from Lamium album flowers. Phytochemistry 1995, 38, 997–1001. [Google Scholar] [CrossRef]
- Van Heerden, F.R.; Brandt, E.V.; Ferreira, D.; Roux, D.G. Metabolites from the purple heartwoods of the Mimosoideae Part 4. Acacia fasciculifera F.Muell. ex Benth. Fasciculiferin, fasciluriferol, and the synthesis of 7-aryl and 7-flavanyl-peltogynoids.”. J. Chem Soc. Perkin Trans. 1981, I, 2483–2490. [Google Scholar]
- Maciel, M.A.; Pinto, A.C.; Arruda, A.C; Pamplona, S.G.; Vanderlinde, F.A.; Lapa, A.J.; Echevarria, A.; Grynberg, N.F.; Côlus, I.M.; Farias, R.A.; Luna Costa, A.M.; Rao, V.S. Ethnopharmacology, phytochemistry and pharmacology: a successful combination in the study of Croton cajucara. J. Ethnopharmacol. 2000, 70, 41–55. [Google Scholar] [CrossRef]
- Murillo, R.M.; Jakupovic, J.; Rivera, J.; Castro, V.H. Diterpenes and other constituents from Croton draco (Euphorbiaceae). Rev. Biol. Trop. 2001, 49, 259–264. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zou, G.-A.; Su, Z.-H.; Zhang, H.-W.; Wang, Y.; Yang, J.-S.; Zou, Z.-M. Flavonoids from the Stems of Croton caudatus Geisel. var. tomentosus Hook. Molecules 2010, 15, 1097-1102. https://doi.org/10.3390/molecules15031097
Zou G-A, Su Z-H, Zhang H-W, Wang Y, Yang J-S, Zou Z-M. Flavonoids from the Stems of Croton caudatus Geisel. var. tomentosus Hook. Molecules. 2010; 15(3):1097-1102. https://doi.org/10.3390/molecules15031097
Chicago/Turabian StyleZou, Guo-An, Zhi-Heng Su, Hong-Wu Zhang, Yuan Wang, Jun-Shan Yang, and Zhong-Mei Zou. 2010. "Flavonoids from the Stems of Croton caudatus Geisel. var. tomentosus Hook" Molecules 15, no. 3: 1097-1102. https://doi.org/10.3390/molecules15031097
APA StyleZou, G.-A., Su, Z.-H., Zhang, H.-W., Wang, Y., Yang, J.-S., & Zou, Z.-M. (2010). Flavonoids from the Stems of Croton caudatus Geisel. var. tomentosus Hook. Molecules, 15(3), 1097-1102. https://doi.org/10.3390/molecules15031097