The Influence of α-, β-, and γ-Melanocyte Stimulating Hormone on Acetaminophen Induced Liver Lesions in Male CBA Mice
Abstract
:1. Introduction

2. Results and Discussion
2.1. Plasma transaminase activity
| Substance | Mean | SD | Median | P value |
|---|---|---|---|---|
| Control 0.9% NaCl | 6767.3 | 6468.6 | 5434.0 | |
| α-MSH 6 × 10-8 mol/kg* | 4880.4 | 3891.4 | 3691.0 | 0.998 |
| α-MSH 3 × 10-7 mol/kg* | 2613.9 | 1114.9 | 2368.5 | 0.999 |
| α-MSH 6 × 10-7 mol/kg* | 1418.5 | 1228.4 | 905.5 | 0.398 |
| α-MSH 1.5 × 10-6 mol/kg* | 539.5 | 459.6 | 414.0 | 0.017 |
| α-MSH 3 × 10-6 mol/kg | 8845.0 | 8311.0 | 7788.0 | 0.995 |
| β-MSH 5 × 10-8 mol/kg | 881.9 | 900.4 | 669.5 | 0.145 |
| β-MSH 1 × 10-7 mol/kg | 182.0 | 49.3 | 181.5 | 0.003 |
| β-MSH 2 × 10-7 mol/kg | 578.5 | 268.6 | 446.0 | 0.030 |
| β-MSH 4 × 10-7 mol/kg | 894.4 | 954.4 | 459.0 | 0.070 |
| γ-MSH 5 × 10-8 mol/kg | 252.1 | 197.9 | 173.5 | 0.004 |
| γ-MSH 1 × 10-7 mol/kg | 135.3 | 17.6 | 138.0 | 0.003 |
| γ-MSH 2 × 10-7 mol/kg | 197.4 | 63.2 | 175.0 | 0.003 |
| γ-MSH 4 × 10-7 mol/kg | 506.3 | 344.7 | 473.5 | 0.023 |
| Substance | Mean | SD | Median | P value |
|---|---|---|---|---|
| Control 0.9% NaCl | 9550.0 | 9213.2 | 7321.5 | |
| α-MSH 6 × 10-8 mol/kg* | 8983.7 | 4654.4 | 7901.0 | 0.962 |
| α-MSH 3 × 10-7 mol/kg* | 4292.0 | 1422.1 | 3945.0 | 0.999 |
| α-MSH 6 × 10-7 mol/kg* | 2671.3 | 2235.1 | 2167.5 | 0.390 |
| α-MSH 1.5 × 10-6 mol/kg* | 585.8 | 1424.1 | 89.0 | 0.012 |
| α-MSH 3 × 10-6 mol/kg | 16853.0 | 10676.0 | 18100.0 | 0.397 |
| β-MSH 5 × 10-8 mol/kg | 2766.5 | 2693.6 | 2119.0 | 0.270 |
| β-MSH 1 × 10-7 mol/kg | 55.3 | 17.7 | 56.5 | 0.003 |
| β-MSH 2 × 10-7 mol/kg | 811.4 | 440.6 | 808.5 | 0.003 |
| β-MSH 4 × 10-7 mol/kg | 2368.5 | 3033.9 | 1322.0 | 0.041 |
| γ-MSH 5 × 10-8 mol/kg | 107.8 | 52.0 | 82.0 | 0.003 |
| γ-MSH 1 × 10-7 mol/kg | 75.8 | 23.5 | 82.5 | 0.003 |
| γ-MSH 2 × 10-7 mol/kg | 85.1 | 37.5 | 80.5 | 0.003 |
| γ-MSH 4 × 10-7 mol/kg | 340.4 | 216.0 | 340.0 | 0.003 |
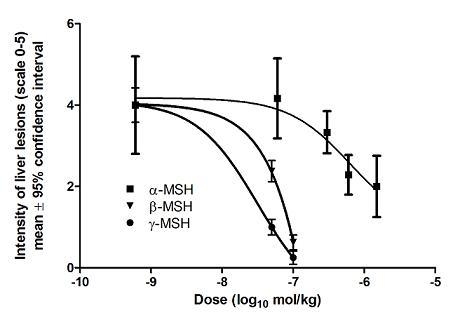
2.2. Histopathological analysis of liver lesions
| Substance | Mean | SD | Median | P value |
|---|---|---|---|---|
| Control 0.9% NaCl | 4.00 | 1.20 | 4.5 | |
| α-MSH 6 × 10-8 mol/kg* | 4.17 | 0.98 | 4.5 | 0.999 |
| α-MSH 3 × 10-7 mol/kg* | 3.33 | 0.52 | 3.0 | 0.5600 |
| α-MSH 6 × 10-7 mol/kg* | 2.29 | 0.49 | 2.0 | 0.0320 |
| α-MSH 1.5 × 10-6 mol/kg* | 2.00 | 0.76 | 2.0 | 0.0178 |
| α-MSH 3 × 10-6 mol/kg | 5.00 | 0.0 | 5.0 | 0.456 |
| β-MSH 5 × 10-8 mol/kg | 2.38 | 0.74 | 2.5 | 0.0471 |
| β-MSH 1 × 10-7 mol/kg | 0.63 | 0.52 | 1.0 | 0.0021 |
| β-MSH 2 × 10-7 mol/kg | 1.00 | 1.07 | 1.0 | 0.0043 |
| β-MSH 4 × 10-7 mol/kg | 2.00 | 1.31 | 2.0 | 0.0410 |
| γ-MSH 5 × 10-8 mol/kg | 1.00 | 0.54 | 1.0 | 0.0023 |
| γ-MSH 1 × 10-7 mol/kg | 0.25 | 0.46 | 0.0 | 0.0019 |
| γ-MSH 2 × 10-7 mol/kg | 0.25 | 0.46 | 0.0 | 0.0019 |
| γ-MSH 4 × 10-7 mol/kg | 0.50 | 0.76 | 0.0 | 0.0026 |
| Substance | Histopathology score≥ 3 | P value |
|---|---|---|
| Control 0.9% NaCl | 7/8 (87.5%) | |
| α-MSH 6 × 10-8 mol/kg | 6/6 (100%) | 0.999 |
| α-MSH 3 × 10-7 mol/kg | 6/6 (100%) | 0.560 |
| α-MSH 6 × 10-7 mol/kg | 2/7 (28.6%) | 0.032 |
| α-MSH 1.5 × 10-6 mol/kg | 2/8 (25.0%) | 0.018 |
| α-MSH 3 × 10-6 mol/kg | 8/8 (100%) | 0.999 |
| β-MSH 5 × 10-8 mol/kg | 4/8 (50.0%) | 0.047 |
| β-MSH 1 × 10-7 mol/kg | 0/8 (0.00%) | 0.002 |
| β-MSH 2 × 10-7 mol/kg | 0/8 (0.00%) | 0.004 |
| β-MSH 4 × 10-7 mol/kg | 3/8 (37.5%) | 0.041 |
| γ-MSH 5 × 10-8 mol/kg | 0/8 (0.00%) | 0.002 |
| γ-MSH 1 × 10-7 mol/kg | 0/8 (0.00%) | 0.002 |
| γ-MSH 2 × 10-7 mol/kg | 0/8 (0.00%) | 0.002 |
| γ-MSH 4 × 10-7 mol/kg | 0/8 (0.00%) | 0.003 |

3. Experimental
3.1. Animals
3.2. Substances
3.3. Treatment regimen
3.4. Plasma transaminase activity
3.5. Histopathological analysis of liver lesions
- 0.
- no lesions
- 1.
- minimal lesions (individual or a few necrotic cells)
- 2.
- mild lesions (10–25% necrotic cells or mild diffuse degenerative lesions)
- 3.
- moderate lesions (25–40% necrotic or degenerative cells)
- 4.
- marked lesions (40–50% necrotic or degenerative cells)
- 5.
- severe lesions (more than 50% necrotic or degenerative cells)
3.6. Statistical analysis
4. Conclusions
Acknowledgements
- Sample Availability: Not available.
References
- Kumar, V.; Abbas, A.K.; Fausto, N.; Mitchell, R.N. Robbins Basic Pathology; Saunders: Philadelphia, PA, USA, 2007; pp. 639–648. [Google Scholar]
- Lange, C.; Sarrazin, C. New agents for treatment. In Hepatology: A Clinical Textbook; Mauss, S., Berg, T., Rockstroh, J., Sarrazin, C., Wedemeyer, H., Eds.; Flying Publisher: Düsseldorf, Germany, 2009; pp. 211–244. [Google Scholar]
- Brzoska, T.; Luger, T.A.; Maaser, C.; Abels, C.; Böhm, M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr. Rev. 2008, 29, 581–602. [Google Scholar] [CrossRef]
- Getting, S.J. Targeting melanocortin receptors as potential novel therapeutics. Pharmacol. Ther. 2006, 111, 1–15. [Google Scholar] [CrossRef]
- Lee, D.J.; Biros, D.J.; Taylor, A.W. Injection of an alpha-melanocyte stimulating hormone expression plasmid is effective in suppressing experimental autoimmune uveitis. Int. Immunopharmacol. 2009, 9, 1079–1086. [Google Scholar] [CrossRef]
- Kokot, A.; Sindrilaru, A.; Schiller, M.; Sunderkötter, C.; Kerkhoff, C.; Eckes, B.; Scharffetter-Kochanek, K.; Luger, T.A.; Böhm, M. alpha-melanocyte-stimulating hormone suppresses bleomycin-induced collagen synthesis and reduces tissue fibrosis in a mouse model of scleroderma: Melanocortin peptides as a novel treatment strategy for scleroderma? Arthritis Rheum. 2009, 60, 592–603. [Google Scholar] [CrossRef]
- Getting, S.J.; Riffo-Vasquez, Y.; Pitchford, S.; Kaneva, M.; Grieco, P.; Page, C.P.; Perretti, M.; Spina, D. A role for MC3R in modulating lung inflammation. Pulm. Pharmacol. Ther. 2008, 21, 866–873. [Google Scholar] [CrossRef]
- Lasaga, M.; Debeljuk, L.; Durand, D.; Scimonelli, T.N.; Caruso, C. Role of alpha-melanocyte stimulating hormone and melanocortin 4 receptor in brain inflammation. Peptides 2008, 29, 1825–1835. [Google Scholar] [CrossRef]
- Böhm, M.; Luger, T.A.; Tobin, D.J.; Garcia-Borron, J.G. Melanocortin receptor ligands: New horizons for skin biology and clinical dermatology. J. Invest. Dermatol. 2006, 126, 1966–1975. [Google Scholar]
- Scholzen, T.E.; Sunderkotter, C.; Kalden, D.H.; Brzoska, T.; Fastrich, M.; Fisbeck, T.; Armstrong, C.A.; Ansel, J.C.; Luger, T.A. α-Melanocyte stimulating hormone prevents lipopolysaccharide-induced vasculitis by down-regulating endothelial cell adhesion molecule expression. Endocrinology 2003, 144, 360–370. [Google Scholar]
- Spencer, J.D.; Schallreuter, K.U. Regulation of pigmentation in human epidermal melanocytes by functional high-affinity β-melanocyte-Stimulating hormone/melanocortin-4 receptor signaling. Endocrinology 2009, 150, 1250–1258. [Google Scholar]
- Bertolini, A.; Tacchi, R.; Vergoni, A.V. Brain effects of melanocortins. Pharmacol. Res. 2009, 59, 13–47. [Google Scholar] [CrossRef]
- Wang, C.H.; Lee, T.H.; Lu, C.N.; Chou, W.Y.; Hung, K.S.; Concejero, A.M.; Jawan, B. Electroporative alpha-MSH gene transfer attenuates thioacetamide-induced murine hepatic fibrosis by MMP and TIMP modulation. Gene Ther. 2006, 13, 1000–1009. [Google Scholar] [CrossRef]
- Lee, T.H.; Jawan, B.; Chou, W.Y.; Lu, C.N.; Wu, C.L.; Kuo, H.M.; Concejero, A.M.; Wang, C.H. Alpha-melanocyte-stimulating hormone gene therapy reverses carbon tetrachloride induced liver fibrosis in mice. J. Gene Med. 2006, 8, 764–772. [Google Scholar] [CrossRef]
- Wang, C.H.; Jawan, B.; Lee, T.H.; Hung, K.S.; Chou, W.Y.; Lu, C.N.; Liu, J.K.; Chen, Y.J. Single injection of naked plasmid encoding alpha-melanocyte-stimulating hormone protects against thioacetamide-induced acute liver failure in mice. Biochem. Biophys. Res. Commun. 2004, 322, 153–161. [Google Scholar] [CrossRef]
- Chiao, H.; Foster, S.; Thomas, R.; Lipton, J.; Star, R.A. Alpha-melanocyte-stimulating hormone reduces endotoxin-induced liver inflammation. J. Clin. Invest. 1996, 97, 2038–2044. [Google Scholar] [CrossRef]
- Nelson, S.D.; Bruschi, S.A. Mechanisms of acetaminophen-induced liver disease. In Drug-Induced liver disease; Kaplowitz, N., DeLeve, L.D., Eds.; Informa Healthcare: New York, NY, USA, 2007; pp. 353–388. [Google Scholar]
- Timbrel, J.A. Principles of Biochemical Toxicology; Informa Healthcare: New York, NY, USA, 2009; pp. 313–321. [Google Scholar]
- Guarner, F.; Boughton-Smith, N.K.; Blackwell, G.J.; Moncada, S. Reduction by prostacyclin of acetaminophen-induced liver toxicity in the mouse. Hepatology 1988, 8, 248–253. [Google Scholar] [CrossRef]
- Čulo, F.; Renić, M.; Sabolović, D.; Radoš, M.; Bilić, A.; Jagić, V. Ketoconazole inhibits acetaminophen-induced hepatotoxicity in mice. Eur. J. Gastroenterol. Hepatol. 1995, 7, 757–762. [Google Scholar]
- Silva, V.M.; Chen, C.; Hennig, G.E.; Whiteley, H.E.; Manautou, E.J. Changes in susceptibility to acetaminophen-induced liver injury by the organic anion indocyanine green. Food. Chem. Tox. 2001, 3, 271–278. [Google Scholar]
- Manautou, J.E.; Silva, V.M.; Hennig, G.E.; Whiteley, H.E. Repeated dosing with the peroxisome proliferation clofibrate decreases the toxicity of model hepatotoxic agents in male mice. Toxicology 1998, 172, 1–10. [Google Scholar]
- Turčić, P.; Bradamante, M.; Houra, K.; Štambuk, N.; Kelava, T.; Konjevoda, P.; Kazazić, S.; Vikić-Topić, D.; Pokrić, B. Effects of α-melanocortin enantiomers on acetaminophen induced hepatotoxicity in CBA mice. Molecules 2009, 14, 5017–5026. [Google Scholar]
- Kastin, A.J.; Pan, W. Peptides and hormesis. Crit. Rev. Toxicol. 2008, 38, 629–631. [Google Scholar]
- Kilty, C.G.; Keenan, J.; Shaw, M. Histologically defined biomarkers in toxicology. Expert Opin. Drug Saf. 2005, 6, 207–215. [Google Scholar]
- Patrick, G. Instant Notes Medicinal Chemistry; Bios: Oxford, UK, 2001; pp. 61–73. [Google Scholar]
- Talarida, R.J. Drug Synergism and Dose-effect Analysis; Chapman & Hall/CRC: Boca Raton, FL, USA, 2000; pp. 21–40. [Google Scholar]
- Muceniece, R.; Zvejniece, L.; Kirjanova, O.; Liepinsh, E.; Krigere, L.; Baumane, L.; Kalvinsh, I.; Wikberg, J.E.S.; Dambrova, M. β- and γ-melanocortins inhibit lipopolysaccharide induced nitric oxide production in mice brain. Brain Res. 2004, 995, 7–13. [Google Scholar] [CrossRef]
- Ping-Lim, S.; Andrews, F.J.; O'Brien, P.E. Misoprostol protection against acetaminophen-induced hepatotoxicity in the rat. Dig. Dis. Sci. 1994, 39, 1249–1256. [Google Scholar] [CrossRef]
- Newsome, P.N.; Plevris, J.N.; Nelson, L.N.; Hayes, P.C. Animal models of fulminant hepatic failure: A critical evaluation. Liver Transpl. 2000, 6, 21–31. [Google Scholar]
- Rowe, P. Essential Statistics for the Pharmaceutical Sciences; Wiley: Chichester, UK, 2007; pp. 195–242. [Google Scholar]
- KyensLab Inc: Tokyo, Japan. Available online: http://www.kyenslab.com/ accessed 17 September 2009.
- GraphPad Software; San Diego, CA, USA. Available online: http://www.graphpad.com/ accessed 17 September 2009.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Blagaić, V.; Houra, K.; Turčić, P.; Štambuk, N.; Konjevoda, P.; Boban-Blagaić, A.; Kelava, T.; Kos, M.; Aralica, G.; Čulo, F. The Influence of α-, β-, and γ-Melanocyte Stimulating Hormone on Acetaminophen Induced Liver Lesions in Male CBA Mice. Molecules 2010, 15, 1232-1241. https://doi.org/10.3390/molecules15031232
Blagaić V, Houra K, Turčić P, Štambuk N, Konjevoda P, Boban-Blagaić A, Kelava T, Kos M, Aralica G, Čulo F. The Influence of α-, β-, and γ-Melanocyte Stimulating Hormone on Acetaminophen Induced Liver Lesions in Male CBA Mice. Molecules. 2010; 15(3):1232-1241. https://doi.org/10.3390/molecules15031232
Chicago/Turabian StyleBlagaić, Vladimir, Karlo Houra, Petra Turčić, Nikola Štambuk, Paško Konjevoda, Alenka Boban-Blagaić, Tomislav Kelava, Marina Kos, Gorana Aralica, and Filip Čulo. 2010. "The Influence of α-, β-, and γ-Melanocyte Stimulating Hormone on Acetaminophen Induced Liver Lesions in Male CBA Mice" Molecules 15, no. 3: 1232-1241. https://doi.org/10.3390/molecules15031232