Determination of the Volatile Constituents in Radix Flemingiae Philippinensis by GC-MS and a Heuristic Evolving Latent Projection Method
Abstract
:Introduction
Results and Discussion
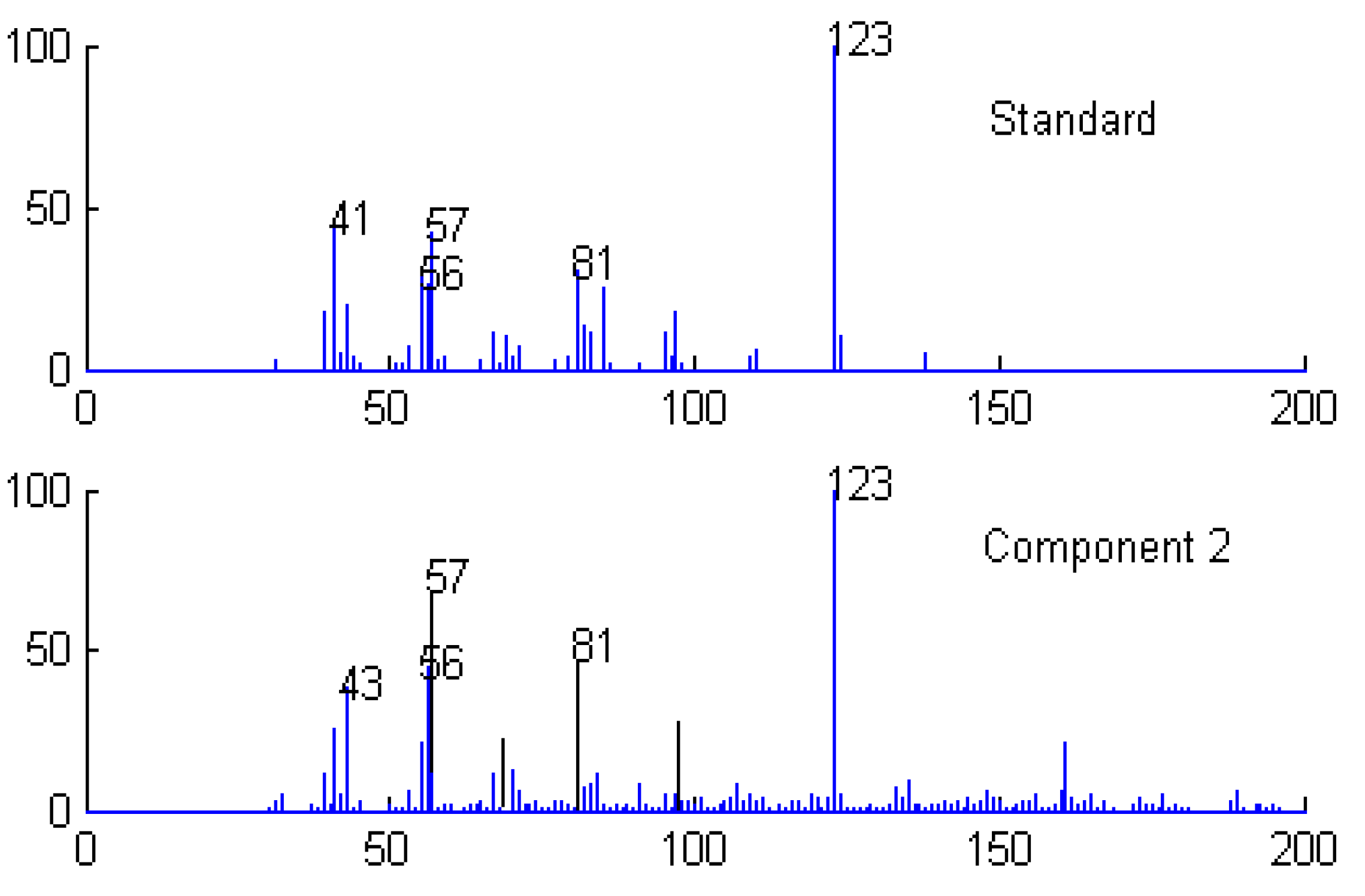
Qualitative analysis



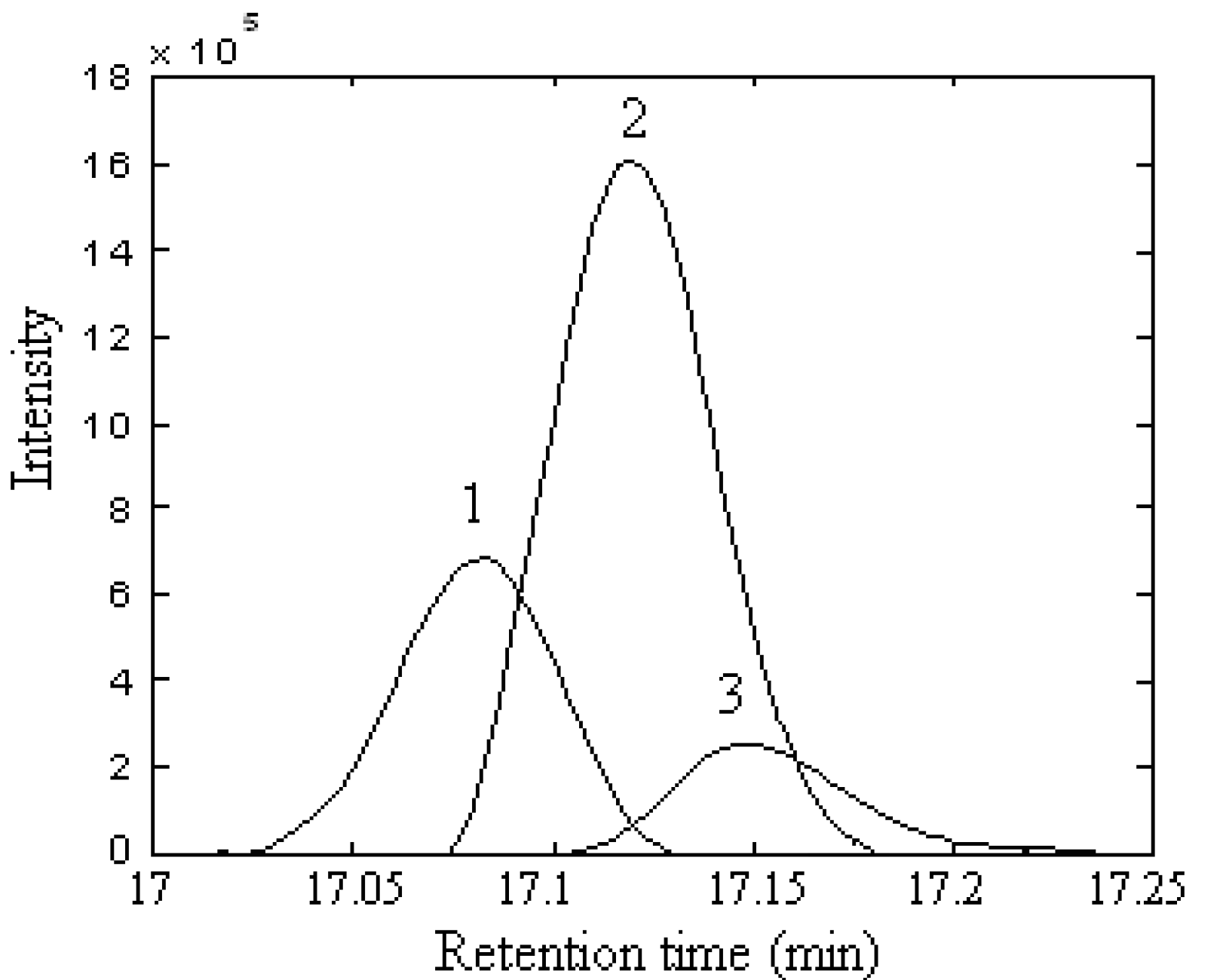


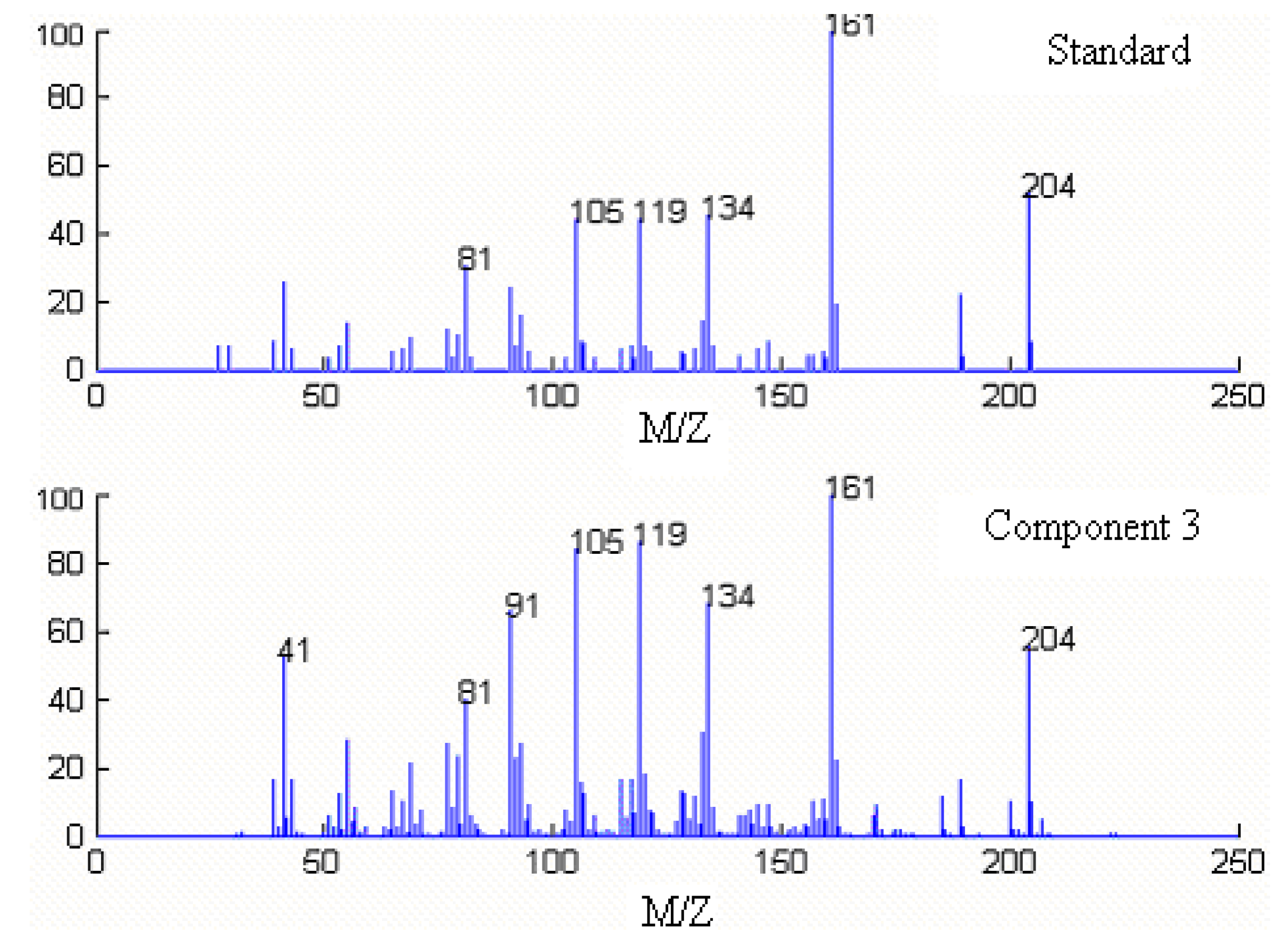
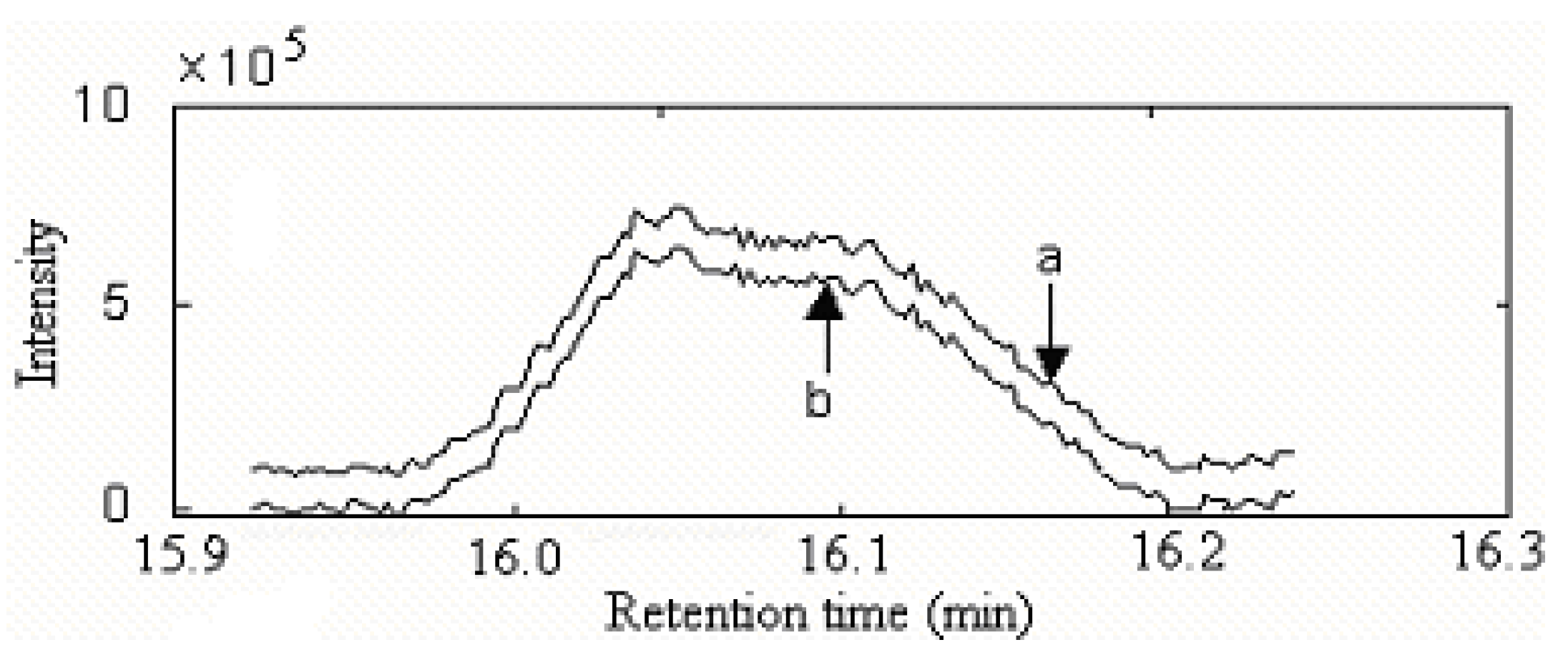

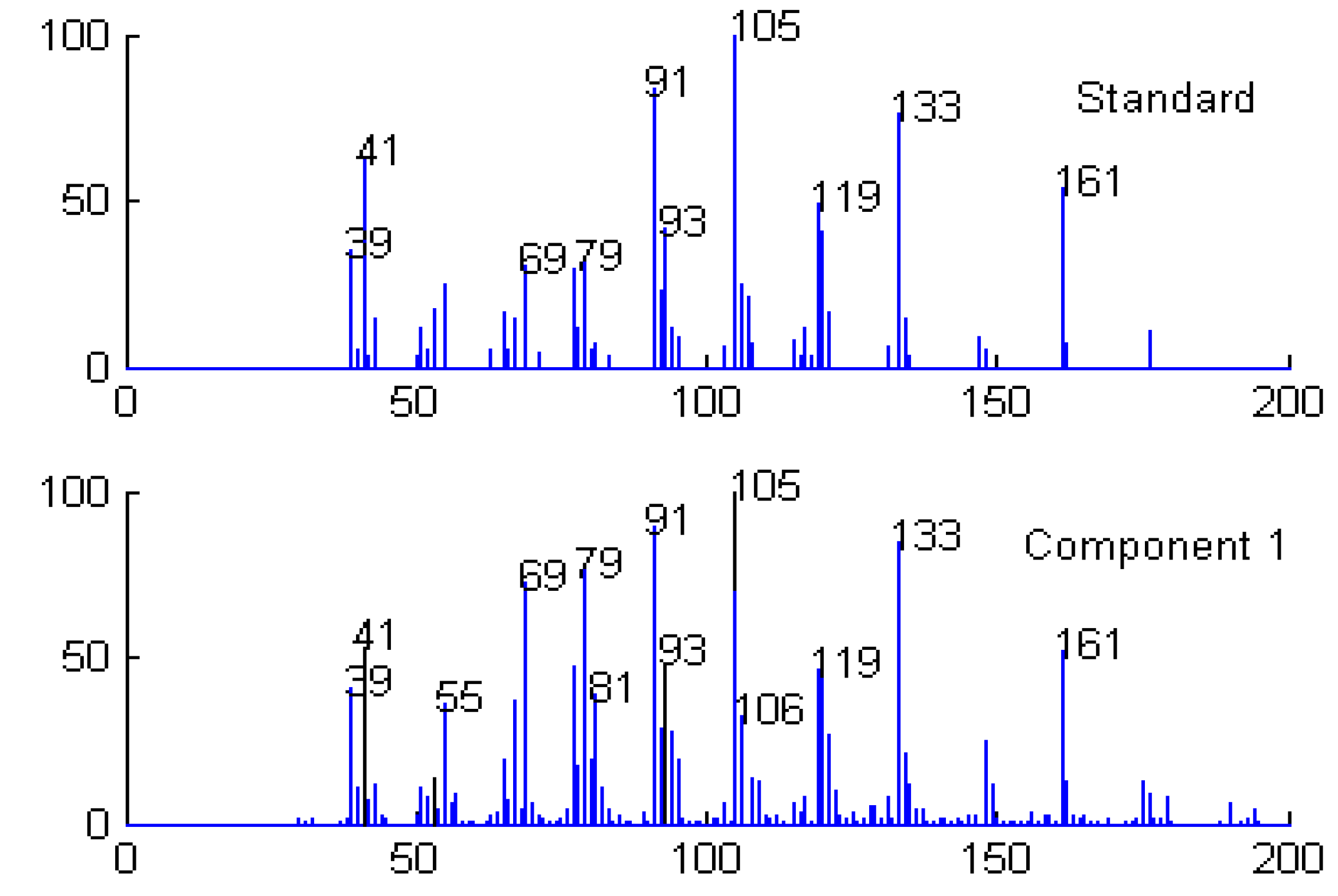
| Series No. | Retention time (min) | Compound name | Molecular formula | Relative content (%) | |
|---|---|---|---|---|---|
| 1 | 3.653 | Hexanal | C6H12O | 0.29 | |
| 2 | 6.368 | 2-Amylfuran | C9H14O | 0.08 | |
| 3 | 7.979 | Linalool | C10H18O | 0.04 | |
| 4 | 8.682 | L-camphor | C10H16O | 0.05 | |
| 5 | 9.267 | p-Menth-1-en-4-ol | C10H18O | 0.04 | |
| 6 | 9.419 | p-Menth-1-en-8-ol | C10H18O | 0.13 | |
| 7 | 10.549 | Nonanoic acid | C9H18O2 | 0.06 | |
| 8 | 11.065 | Bornyl acetate | C12H20O2 | 0.08 | |
| 9 | 11.294 | 2,3,4,5-Tetramethyltricyclo[3.2.1.02,7]oct-3-ene | C12H 18 | 0.11 | |
| 10 | 12.750 | α-Cubebene | C15H24 | 0.13 | |
| 11 | 12.929 | α-Longipinene | C15H24 | 0.11 | |
| 12 | 13.035 | Di-epi-α-Cedrene | C15H24 | 0.04 | |
| 13 | 13.330 | Ylangene | C15H24 | 1.22 | |
| 14 | 13.476 | Longicyclene | C15H24 | 2.49 | |
| 15 | 13.675 | Copaene | C15H24 | 0.06 | |
| 16 | 14.315 | Longofolene | C15H24 | 5.20 | |
| 17 | 14.560 | β-Caryophyllene | C15H24 | 12.60 | |
| 18 | 14.744 | Germacrene D | C15H24 | 0.30 | |
| 19 | 15.057 | (Z)-β-Farnesene | C15H24 | 0.47 | |
| 20 | 15.232 | 2,4a,5,6,7,8,9,9a-Octahydro-3,5,5-trimethyl-9-methylene 1 H-benzocycloheptene | C15H24 | 0.52 | |
| 21 | 15.398 | α-Caryophyllene | C15H24 | 0.73 | |
| 22 | 15.665 | Dihydrocurcumene | C15H24 | 0.19 | |
| 23 | 15.812 | α-Curcumene | C15H24 | 1.20 | |
| 24 | 15.858 | Eremophilene | C15H24 | 0.56 | |
| 25 | 16.037 | 2-Cyclopropylidene-1,7,7-trimethylbicylo-[2,2,1]- heptane | C13H20 | 0.56 | |
| 26 | 16.142 | 3,3,5,5-Tetramethylcyclohexanol | C10H20O | 0.59 | |
| 27 | 16.153 | Longifolene-(V4) | C15H24 | 0.64 | |
| 28 | 16.288 | α-Guaiene | C15H24 | 0.28 | |
| 29 | 16.419 | Patchoulene | C15H24 | 0.33 | |
| 30 | 16.551 | α-Muurolene | C15H24 | 1.69 | |
| 31 | 16.721 | β-Himachalene | C15H24 | 1.59 | |
| 32 | 17.092 | Calamenene | C15H22 | 1.85 | |
| 33 | 17.133 | δ-Cadinene | C15H24 | 2.13 | |
| 34 | 17.217 | β-Cadinene | C15H24 | 0.90 | |
| 35 | 17.538 | Acoradiene | C15H24 | 0.96 | |
| 36 | 17.693 | 3-Bromomethyl- 1,1-dimethyl-1 H-indene | C15H22 | 3.83 | |
| 37 | 18.134 | Nerolidol | C15H26O | 1.57 | |
| 38 | 18.255 | Cadala-1(10),3,8-triene | C15H22 | 0.26 | |
| 39 | 18.710 | Caryophyllenyl alcohol | C15H26O | 0.18 | |
| 40 | 19.076 | Caryophyllene oxide | C15H24O | 1.37 | |
| 41 | 19.772 | Cedrol | C15H26O | 1.94 | |
| 42 | 19.969 | Bulnesol | C15H26O | 0.31 | |
| 43 | 20.442 | Epiglobulol | C15H26O | 0.36 | |
| 44 | 20.611 | Cubenol | C15H26O | 2.67 | |
| 45 | 20.996 | ι-Cadinol | C15H26O | 0.50 | |
| 46 | 21.090 | δ-Cadinol | C15H26O | 0.75 | |
| 47 | 21.196 | Torreyol | C15H26O | 0.89 | |
| 48 | 21.377 | β-Selinenol | C15H26O | 2.33 | |
| 49 | 21.510 | α-Eudesmol | C15H26O | 0.27 | |
| 50 | 22.025 | Cadalene | C15H18 | 1.34 | |
| 51 | 22.185 | Hedycaryol | C15H26O | 0.11 | |
| 52 | 22.916 | Peruviol | C15H26O | 0.71 | |
| 53 | 25.654 | Farnesol isomer | C15H26O | 31.33 | |
| 54 | 26.361 | cis-Farnesal | C15H24O | 0.66 | |
| 55 | 33.868 | Hexadecanoic acid | C16H32O2 | 1.12 | |
Quantitative analysis
Experimental
Apparatus
Materials
Extraction of the essential oil
Gas chromatography–mass spectrometry
Data analysis
Conclusions
Acknowledgements
- Samples Availability: Samples of the extracts from Radix Flemingiae Philippinensis are available from the authors.
References and Notes
- Wen, K.C.; Huang, C.Y.; Lu, F.L. Determination of baicalin and puerarin in traditional Chinese medicinal preparations by high-performance liquid chromatography. J. Chromatogr. A 1993, 631, 241–250. [Google Scholar] [CrossRef]
- Chen, H.H; Sun, C.C.; Tseng, M.P.; Hsu, C.J. A patch test study of 27 crude drugs commonly used in Chinese topical medicaments. Contact Dermatitis 2003, 49, 8–14. [Google Scholar] [CrossRef]
- Wang, H.; Zou, H.; Kong, L.; Ni, J. Analysis of bioactive components in traditional Chinese medicines by molecular biochromatography with alpha1acid glycoprotein stationary phase. J. Basic Clin. Physiol. Pharmacol. 2000, 11, 155–172. [Google Scholar]
- Kulkarni, R.N.; Mallavarapu, G.R.; Baskaran, K.; Ramesh, S.; Kumar, S. Composition of the essential oils of two isomenthone-rich variants of geranium (Pelargonium sp.). Flavour Frag. J. 1998, 13, 389–392. [Google Scholar] [CrossRef]
- Liu, J.H.; Gao, L.X.; Gao, Y.Q.; Huo, X. Study on the chemical constituents of volatile oil from Radix Flemingiae Philippinensis. Chin. Tradit. Pat. Med. 2003, 6, 485–487. [Google Scholar]
- Maeder, M. Evolving factor analysis for the resolution of overlapping chromatographic peaks. Anal. Chem. 1987, 59, 527–530. [Google Scholar] [CrossRef]
- Maeder, M.; Zilian, A. Evolving factor analysis, a new multivariate technique in chromatography. Chemom. Intell. Lab. Syst. 1988, 3, 205–213. [Google Scholar] [CrossRef]
- Malinowski, E.R. Window factor analysis, theoretic derivation and application to flow injection analysis data. J. Chemometr. 1992, 6, 29–40. [Google Scholar] [CrossRef]
- Kvalheim, O.M.; Liang, Y.Z. Heuristic evolving latent projections: Resolving two-way multicomponent data. Part 1. Selectivity, latent projective graph, datascope, local rank and unique resolution. Anal. Chem. 1992, 64, 936–946. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Kvalheim, O.M.; Keller, H.R.; Massart, D.L.; Kiechle, P.; Erni, F. Heuristic evolving latent projections: Resolving two-way multicomponent data. Part 2: Detection and resolution of minor constituents. Anal. Chem. 1992, 64, 946–953. [Google Scholar] [CrossRef]
- Manne, R.; Shen, H.L.; Liang, Y.Z. Subwindow factor analysis. Chemom. Intell. Lab. Syst. 1999, 45, 171–176. [Google Scholar] [CrossRef]
- Shen, H.L.; Manne, R.; Xu, Q.S.; Chen, D.Z.; Liang, Y.Z. Local resolution of hyphenated chromatographic data. Chemom. Intell. Lab. Syst. 1999, 45, 323–330. [Google Scholar] [CrossRef]
- Guo, F.Q.; Liang, Y.Z.; Xu, C.J; Huang, L.F.; Li, X.N. Comparison of the volatile constituents of Artemisia capillaris from different locations by gas chromatography-mass spectrometry and projection method. J. Chromatogr. A 2004, 1054, 73–79. [Google Scholar]
- Xu, X.N.; Tang, Z.H.; Liang, Y.Z.; Zhang, L.X.; Zeng, M.M.; Deng, J.H. Comparison of the volatile constituents of different parts of Cortex magnolia officinalis by GC–MS combined with chemometric resolution method. J.Sep.Sci. 2009, 32, 3466–3472. [Google Scholar] [CrossRef]
- Yi, L.Z.; Yuan, D.L.; Liang, Y.Z.; Xie, P.S.; Zhao, Y. F Ingerprinting alterations of secondary metabolites of tangerine peels during growth by HPLC-DAD and chemometric methods. Anal.Chim.Acta 2009, 649, 43–51. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Committee, Chinese Pharmacopoeia (2000). Publishing House of People’s Health: Beijing, China; Volume Appendix 62.
- Sanchez, F.C.; Rutan, S.C.; Gil Garcia, N.D. Resolution of multicomponent overlapped peaks by the orthogonal projection approach, evolving factor analysis and window factor analysis. Chemom. Intell. Lab. Syst. 1997, 36, 153–164. [Google Scholar] [CrossRef]
- Liang, Y.Z. White, Grey and Black Multicomponent Systems and Their Chemometric Algorithms; Hunan Publishing House of Science and Technology: Changsha, China, 1996. [Google Scholar]
- Li, X.N.; Liang, Y.Z.; Chau, F.T. Smoothing methods applied to dealing with heteroscedastic noise in GC/MS. Chemom. Intell. Lab. Syst. 2002, 63, 139–153. [Google Scholar] [CrossRef]
© 2010 by the authors;
Share and Cite
Xie, J.-W.; Hu, W.; Zhou, Z.-L.; Huang, L.-F.; Wang, Y.-L.; Fang, J.-J.; He, Y.-B. Determination of the Volatile Constituents in Radix Flemingiae Philippinensis by GC-MS and a Heuristic Evolving Latent Projection Method. Molecules 2010, 15, 4055-4066. https://doi.org/10.3390/molecules15064055
Xie J-W, Hu W, Zhou Z-L, Huang L-F, Wang Y-L, Fang J-J, He Y-B. Determination of the Volatile Constituents in Radix Flemingiae Philippinensis by GC-MS and a Heuristic Evolving Latent Projection Method. Molecules. 2010; 15(6):4055-4066. https://doi.org/10.3390/molecules15064055
Chicago/Turabian StyleXie, Jian-Wei, Wei Hu, Zhao-Liang Zhou, Lan-Fang Huang, Yu-Ling Wang, Jian-Jun Fang, and Yun-Biao He. 2010. "Determination of the Volatile Constituents in Radix Flemingiae Philippinensis by GC-MS and a Heuristic Evolving Latent Projection Method" Molecules 15, no. 6: 4055-4066. https://doi.org/10.3390/molecules15064055




