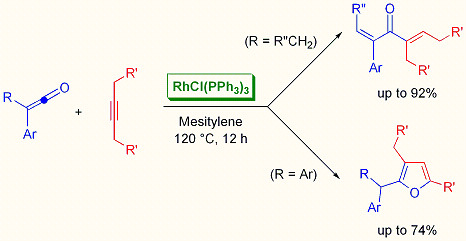
Rhodium-Catalyzed Linear Codimerization and Cycloaddition of Ketenes with Alkynes
Abstract
:1. Introduction
2. Results and Discussion
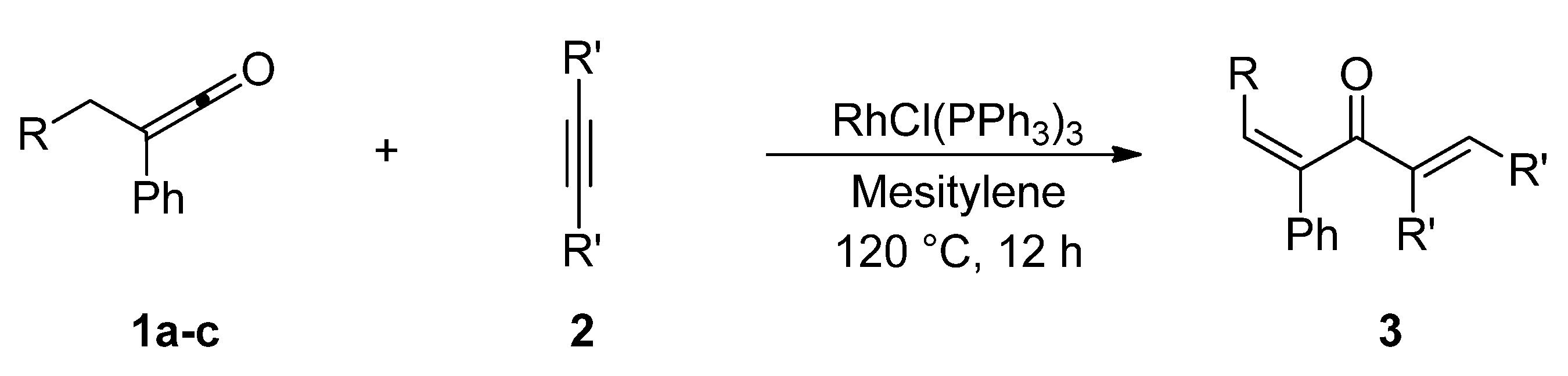
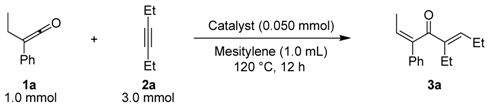 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Yield of 3a (%) a | Entry | Catalyst | Yield of 3a (%) a |
| 1 | RhCl(PPh3)3 | 92 | 7 | RuCl2(PPh3)3 | 1 |
| 2 b | RhCl(PPh3)3 | 5 | 8 c | [RuCl2(CO)3]2 | 0 |
| 3 | RhCl(CO)(PPh3)2 | 46 | 9 | RuH2(PPh3)4 | 0 |
| 4 | RhCl3·3H2O | 26 | 10 | Pd(PPh3)4 | 29 |
| 5 | RhH(PPh3)4 | 2 | 11 | IrCl(CO)(PPh3)2 | 0 |
| 6 | RhH(CO)(PPh3)3 | 0 | |||
| a GLC yield; b RhCl(PPh3)3 (2.0 mol %, 0.020 mmol) for 40 h; c [RuCl2(CO)3]2 (0.025 mmol). | |||||
| Entry | Ketene | Alkyne | Product | Isolated Yield (%) | ||
|---|---|---|---|---|---|---|
| 1 |  | 1a |  | 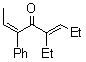 | 3a | 74 (92) b |
| 2a | ||||||
| 2 | 1a | 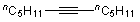 |  | 3b | 68 | |
| 2b | ||||||
| 3 |  | 1b | 2a | 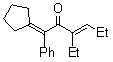 | 3c | 52 |
| 4 |  | 1c | 2a |  | 3d | 40 |
| a Ketene (1.0 mmol), alkyne (3.0 mmol), RhCl(PPh3)3 (0.050 mmol), and mesitylene (1.0 mL) at 120 ºC for 12 h under an argon atmosphere; b GLC yield. | ||||||

 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Yield of 4a (%) a | Entry | Catalyst | Yield of 4a (%) a |
| 1 | RhCl(PPh3)3 | 74 | 7 | [Cp*RuCl2]2 | 0 |
| 2 | RhCl(CO)(PPh3)2 | 2 | 8 | RuCl2(PPh3)3 | 0 |
| 3 | RhCl3·3H2O | 0 | 9 | [RuCl2(CO)3]2 | 0 |
| 4 | [RhCl(cod)]2 | 0 | 10 | IrCl(CO)(PPh3)2 | 0 |
| 5 | [RhCl(C2H4)2]2 | 0 | 11 | Pd(PPh3)4 | 0 |
| 6 | RhH(PPh3)4 | 0 | |||
| a GLC yield. | |||||
| Entry | Ketene | Alkyne | Product | Isolated Yield (%) | |||
|---|---|---|---|---|---|---|---|
| 1 | 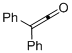 | 1d |  |  | 4a | 64 (74) b | |
| 2a | |||||||
| 2 | 1d | 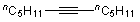 |  | 4b | 43 | ||
| 2b | |||||||
| 3 | 1d |  | 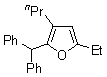 | 4c | 52 | ||
| 2c | |||||||
| 4 | 1d |  | 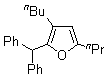 | 4d | 43 | ||
| 2d | |||||||
| 5 |  | 1e | 2a | 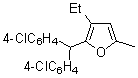 | 4e | 51 (70)b | |
| a Ketene (1.0 mmol), alkyne (3.0 mmol), RhCl(PPh3)3 (0.050 mmol), and mesitylene (1.0 mL) at 120 ºC for 12 h under an argon atmosphere; b GLC yield. | |||||||

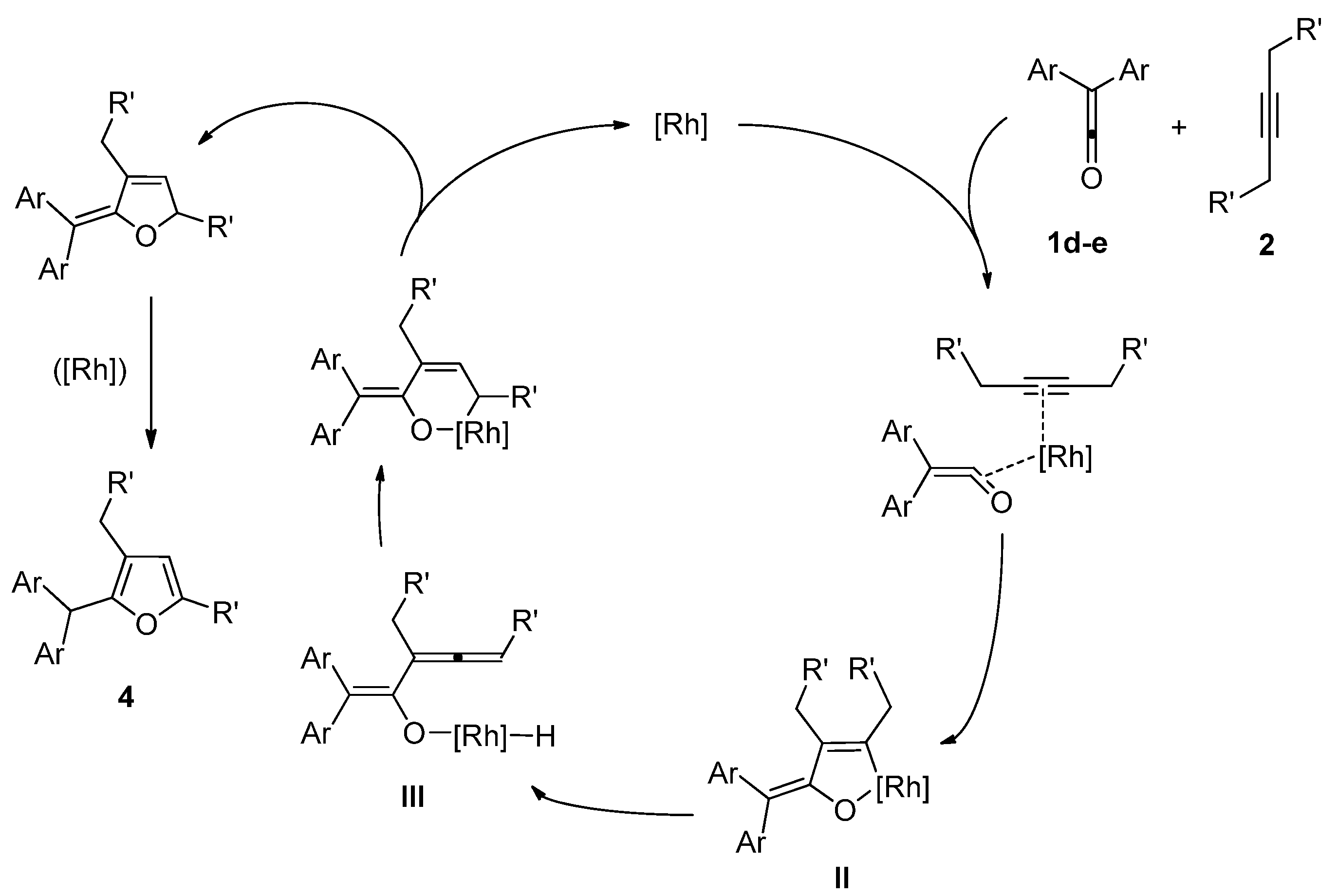
3. Experimental
3.1. General
3.2. Materials
3.3. General procedure for the rhodium-catalyzed reaction of ketenes with alkynes to give dienones and furans
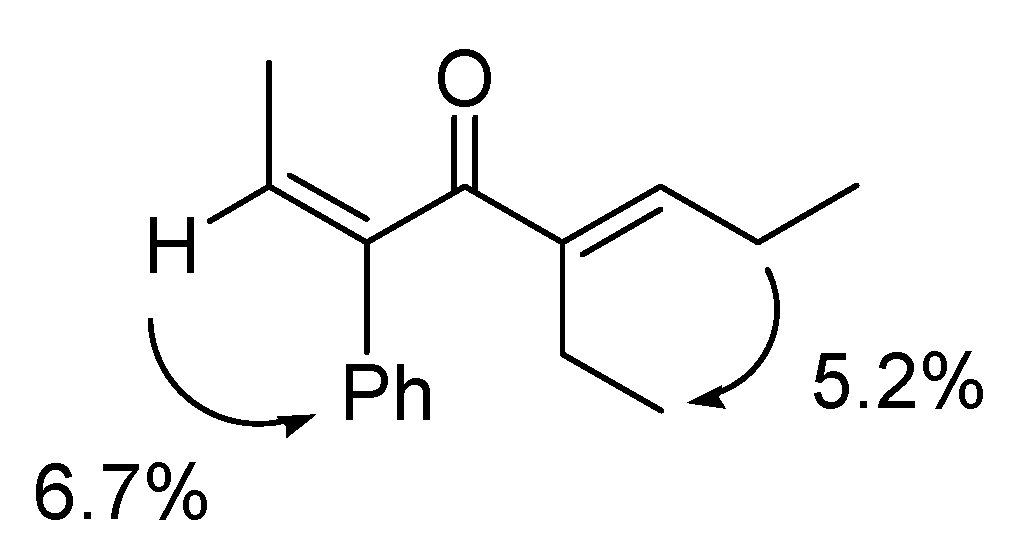

4. Conclusions
Acknowledgements
References and Notes
- The Chemistry of Functional Groups: The Chemistry of Ketenes, Allenes, and Related Compounds Part. 1; Patai, S. (Ed.) John Wiley & Sons: New York, NY, USA, 1980.
- Okumoto, H. Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi, E., Ed.; John Wiley & Sons: New York, NY, USA, 2002; pp. 2655–2662. [Google Scholar]
- Ulrich, H. Cumulenes in Click Reactions; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Geoffroy, G.L.; Bassner, S.L. Interaction of ketenes with organometallic compounds: Ketene, ketenyl, and ketenylidene complexes. Adv. Organomet. Chem. 1988, 28, 1–83. [Google Scholar] [CrossRef]
- Xu, Y.-C.; Challener, C.A.; Dragisich, V.; Brandvold, T.A.; Peterson, G.A.; Wulff, W.D.; Willard, P.G. The generation of 2-vinylcyclopentene-1,3-diones via a five-component coupling in the coordination sphere of chromium. J. Am. Chem. Soc. 1989, 111, 7269–7271. [Google Scholar] [CrossRef]
- Grotjahn, D.B.; Lo, H.C. Fragmentation of 2-pyridyl esters gives both η2(C,O)- and η2 (C,C)-bound ketene ligands on ClIr[P(i-Pr)3]2. Organometallics 1995, 14, 5463–5465. [Google Scholar] [CrossRef]
- Lo, H.C.; Grotjahn, D.B. Selective C−C bond formation on the first ketene−alkyne complexes. J. Am. Chem. Soc. 1997, 119, 2958–2959. [Google Scholar] [CrossRef]
- Grotjahn, D.B.; Bikzhanova, G.A.; Hubbard, J.L. Phosphine loss from bis(phosphine)rhodium(I) η2-(C,O)-diphenylketene complexes leading to η4-(C4) coordination and fluxionality of the ketene. Organometallics 1999, 18, 5614–5619. [Google Scholar] [CrossRef]
- Grotjahn, D.B.; Collins, L.S.B.; Wolpert, M.; Bikzhanova, G.A.; Lo, H.C.; Combs, D.; Hubbard, J.L. First direct structural comparison of complexes of the same metal fragment to ketenes in both C,C- and C,O-Bonding modes. J. Am. Chem. Soc. 2001, 123, 8260–8270. [Google Scholar]
- Grotjahn, D.B.; Hoerter, J.M.; Hubbard, J.L. Double C−H activation during functionalization of phenyl(methyl)ketene on iridium(I) using alkynes. Synthesis of 1,4-dien-3-ones. J. Am. Chem. Soc. 2004, 126, 8866–8867. [Google Scholar] [CrossRef]
- Kondo, T.; Kaneko, Y.; Taguchi, Y.; Nakamura, A.; Okada, T.; Shiotsuki, M.; Ura, Y.; Wada, K.; Mitsudo, T. Rapid ruthenium-catalyzed synthesis of pyranopyrandiones by reconstructive carbonylation of cyclopropenones involving C−C bond cleavage. J. Am. Chem. Soc. 2002, 124, 6824–6825. [Google Scholar]
- Kondo, T.; Taguchi, Y.; Kaneko, Y.; Niimi, M.; Mitsudo, T. Ru- and Rh-catalyzed C-C bond leavage of cyclobutenones: Reconstructive and selective synthesis of 2-pyranones, cyclopentenes, and cyclohexenones. Angew. Chem. Int. Ed. 2004, 43, 5369–5372. [Google Scholar] [CrossRef]
- Kondo, T.; Niimi, M.; Nomura, M.; Wada, K.; Mitsudo, T. Rhodium-catalyzed rapid synthesis of substituted phenols from cyclobutenones and alkynes or alkenes via C–C bond cleavage. Tetrahedron Lett. 2007, 48, 2837–2839. [Google Scholar] [CrossRef]
- Hong, P.; Yamazaki, H.; Sonogashira, K.; Hagihara, N. Rhodium carbonyl cluster catalyzed addition of arenes to diphenylketene and aryl isocyanate. Chem. Lett. 1978, 535–538. [Google Scholar]
- Yamazaki, H.; Hong, P. Organic reactions with rhodium carbonyl cluster catalysts. J. Mol. Catal. 1983, 21, 133–150. [Google Scholar]
- Mitsudo, T.; Kadokura, M.; Watanabe, Y. Palladium complex catalyzed reaction of diphenylketene with terminal acetylenes giving disubstituted acetylenes. Tetrahedron Lett. 1985, 26, 3697–3698. [Google Scholar] [CrossRef]
- Mitsudo, T.; Kadokura, M.; Watanabe, Y. Palladium complex catalyzed synthesis of α,β-unsaturated ketones from alkylphenylketene and aroyl chloride. Tetrahedron Lett. 1985, 26, 5143–5144. [Google Scholar] [CrossRef]
- Mitsudo, T.; Kadokura, M.; Watanabe, Y. Palladium-catalysed reactions of ketenes with allyl acetates or allyl carbonates: novel syntheses of 1,3-dienes and allylated esters. J. Chem. Soc., Chem. Commun. 1986, 1539–1541. [Google Scholar]
- Mitsudo, T.; Kadokura, M.; Watanabe, Y. Palladium-complex-catalyzed reactions of ketenes with allylic carbonates or acetates. Novel syntheses of α-allylated carboxylic esters and 1,3-dienes. J. Org. Chem. 1987, 52, 1695–1699. [Google Scholar] [CrossRef]
- Mitsudo, T.; Kadokura, M.; Watanabe, Y. Novel synthesis of α,β-unsaturated ketones by the palladium-catalyzed arylation of ketenes with aroyl chlorides or the decarbonylative cross-condensation of acyl halides. J. Org. Chem. 1987, 52, 3186–3192. [Google Scholar] [CrossRef]
- Kondo, T.; Tokoro, Y.; Ura, Y.; Wada, K.; Mitsudo, T. Rhodium-Catalyzed decarbonylative coupling reactions of diphenylketene with alkenes. ChemCatChem 2009, 1, 82–84. [Google Scholar] [CrossRef]
- Straus, D.A.; Grubbs, R.H. Preparation and reaction of metal-ketene complexes of zirconium and titanium. J. Am. Chem. Soc. 1982, 104, 5499–5500. [Google Scholar] [CrossRef]
- Huffman, M.A.; Liebeskind, L.S. Insertion of (η5-indeny)cobalt(I) into cyclobutenones: the first synthesis of phenols from isolated vinylketene complexes. J. Am. Chem. Soc. 1990, 112, 8617–8618. [Google Scholar] [CrossRef]
- Anderson, B.A.; Wulff, W.D.; Rheingold, A.L. Characterization of the first η4-vinylketene metal complex from the reaction of a Group 6 Fischer carbene complex and an alkyne. J. Am. Chem. Soc. 1990, 112, 8615–8616. [Google Scholar] [CrossRef]
- Bao, J.; Wulff, W.D.; Dragisich, V.; Wenglowsy, S.; Ball, R.S. Three-component intramolecular two-alkyne annulations of Fischer carbene complexes: new strategies for steroid synthesis. J. Am. Chem. Soc. 1994, 116, 7616–7630. [Google Scholar] [CrossRef]
- Brady, W.T. Halogenated ketenes: valuable intermediates in organic synthesis. Synthesis 1971, 415–422. [Google Scholar] [CrossRef]
- Allen, A.D.; Baigrie, L.M.; Gong, L.; Tidwell, T.T. Cyclopropylketenes: preparation and nucleophilic additions. Can. J. Chem. 1991, 69, 138–145. [Google Scholar] [CrossRef]
- Osborn, J.A.; Jardine, J.F.; Young, J.F.; Wilkinson, G. Tris(triphenylphosphine)halorhodium(I). Inorg. Synth. 1967, 10, 67–71. [Google Scholar]
- McCleverty, J.A.; Wilkinson, G. Chlorocarbonylbis(triphenylphosphine)rhodium and chloro-carbonylbis(triphenylarsine)rhodium. Inorg. Synth. 1966, 8, 214–217. [Google Scholar] [CrossRef]
- Ahmad, N.; Levinson, J.J.; Robinson, S.D.; Uttley, M.F. Hydrido phosphine complexes of rhodium(I). Inorg. Synth. 1990, 28, 81–83. [Google Scholar]
- Wonchoba, E.R.; Parshall, G.W. Complexes of ruthenium, osmium, rhodium, and iridium containing hydride carbonyl, or nitrosyl ligands. Inorg. Synth. 1974, 15, 45–64. [Google Scholar]
- Ent, A.; Onderdelinden, A.L. Di-μ-chlorotetrakis(ethylene)dirhodium(I), 2,4-pentanedionato-bis(ethylene)rhodium(I), and di-μ-chlorotetracarbonyldirhodium(I). Inorg. Synth. 1974, 15, 14–18. [Google Scholar]
- Hallman, P.S.; Stephenson, T.A.; Wilkinson, G. Tetrakis(triphenylphosphine)dichlororuthenium-(II) and tris(triphenylphosphine)dichlororuthenium(II). Inorg. Synth. 1970, 12, 237–240. [Google Scholar] [CrossRef]
- Young, R.; Wilkinson, G. Dihydridotetrakis(triphenylphosphine)ruthenium(II). Inorg. Synth. 1977, 17, 75–77. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3a-d and 4a-e are available from the authors.
© 2010 by the authors;
Share and Cite
Kondo, T.; Niimi, M.; Yoshida, Y.; Wada, K.; Mitsudo, T.-a.; Kimura, Y.; Toshimitsu, A. Rhodium-Catalyzed Linear Codimerization and Cycloaddition of Ketenes with Alkynes. Molecules 2010, 15, 4189-4200. https://doi.org/10.3390/molecules15064189
Kondo T, Niimi M, Yoshida Y, Wada K, Mitsudo T-a, Kimura Y, Toshimitsu A. Rhodium-Catalyzed Linear Codimerization and Cycloaddition of Ketenes with Alkynes. Molecules. 2010; 15(6):4189-4200. https://doi.org/10.3390/molecules15064189
Chicago/Turabian StyleKondo, Teruyuki, Masatsugu Niimi, Yuki Yoshida, Kenji Wada, Take-aki Mitsudo, Yu Kimura, and Akio Toshimitsu. 2010. "Rhodium-Catalyzed Linear Codimerization and Cycloaddition of Ketenes with Alkynes" Molecules 15, no. 6: 4189-4200. https://doi.org/10.3390/molecules15064189
APA StyleKondo, T., Niimi, M., Yoshida, Y., Wada, K., Mitsudo, T.-a., Kimura, Y., & Toshimitsu, A. (2010). Rhodium-Catalyzed Linear Codimerization and Cycloaddition of Ketenes with Alkynes. Molecules, 15(6), 4189-4200. https://doi.org/10.3390/molecules15064189