The Protective Effects of the Active Fraction of Shaofu Zhuyu Decoction on Hydrogen Peroxide-Induced Oxidative Injury in Vascular Smooth Muscle Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of SF-7 on viability of H2O2-injured VSMCs
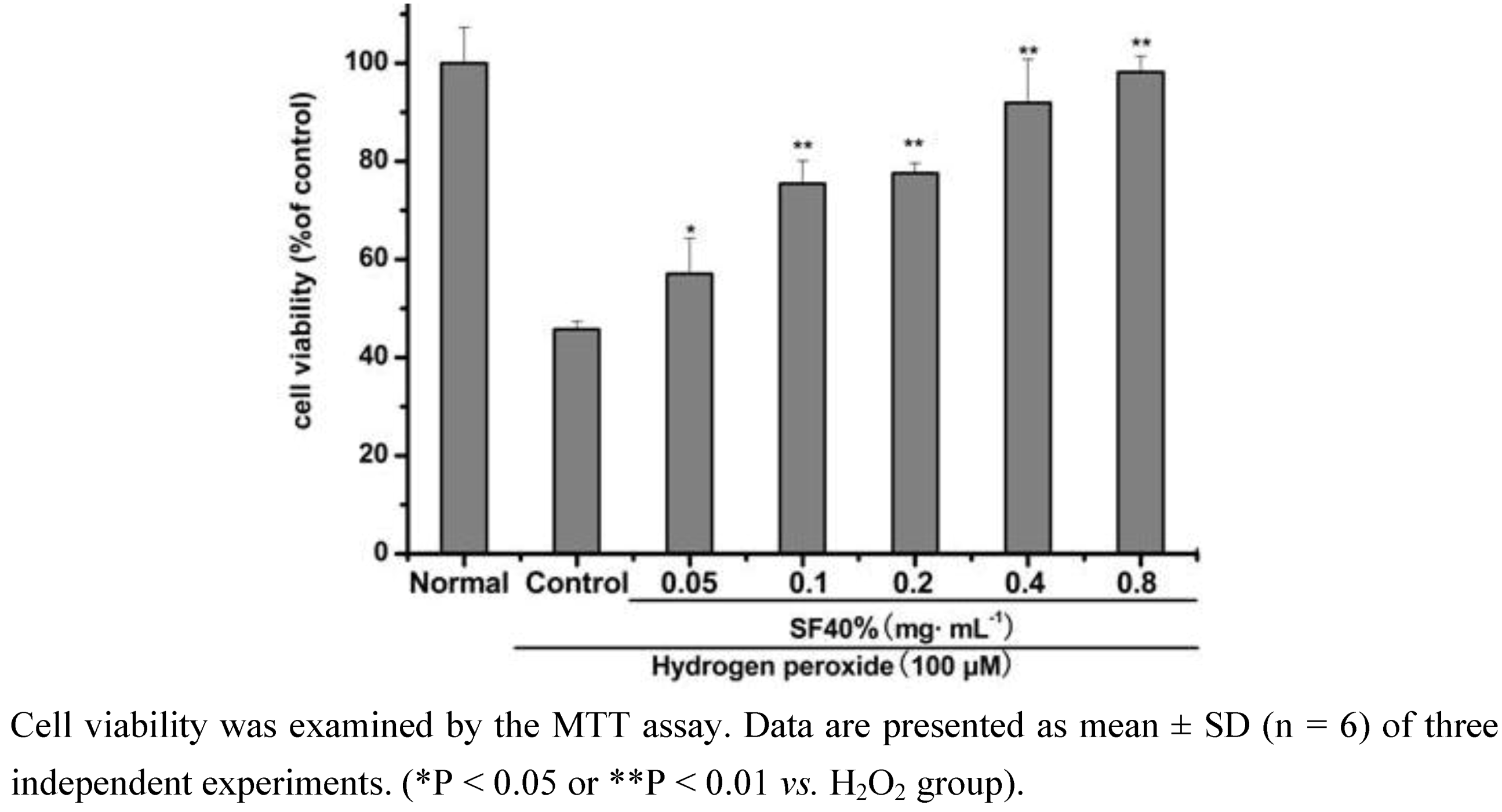
2.2. Effects of SF-7 on LDH leakage, MDA level and SOD activity in H2O2-injured VSMCs
| Group | LDH | MDA | SOD |
|---|---|---|---|
| (% of normal) | |||
| Normal | 100.00 ± 1.70 | 100.21 ± 4.65 | 99.36 ± 4.24 |
| Control | 148.97 ± 18.88 | 146.77 ± 4.23 | 46.28 ± 4.35 |
| 0.1 mg·mL-1 | 145.62 ± 4.88 | 127.83 ± 16.40* | 65.57 ± 2.22** |
| 0.2 mg·mL-1 | 124.00 ± 2.85** | 123.09 ± 12.53** | 76.48 ± 4.43** |
| 0.4 mg·mL-1 | 107.08 ± 6.29** | 119.94 ± 7.23** | 96.31 ± 3.81** |
2.3. Effect of SF-7 on apoptosis in H2O2-injured VSMCs
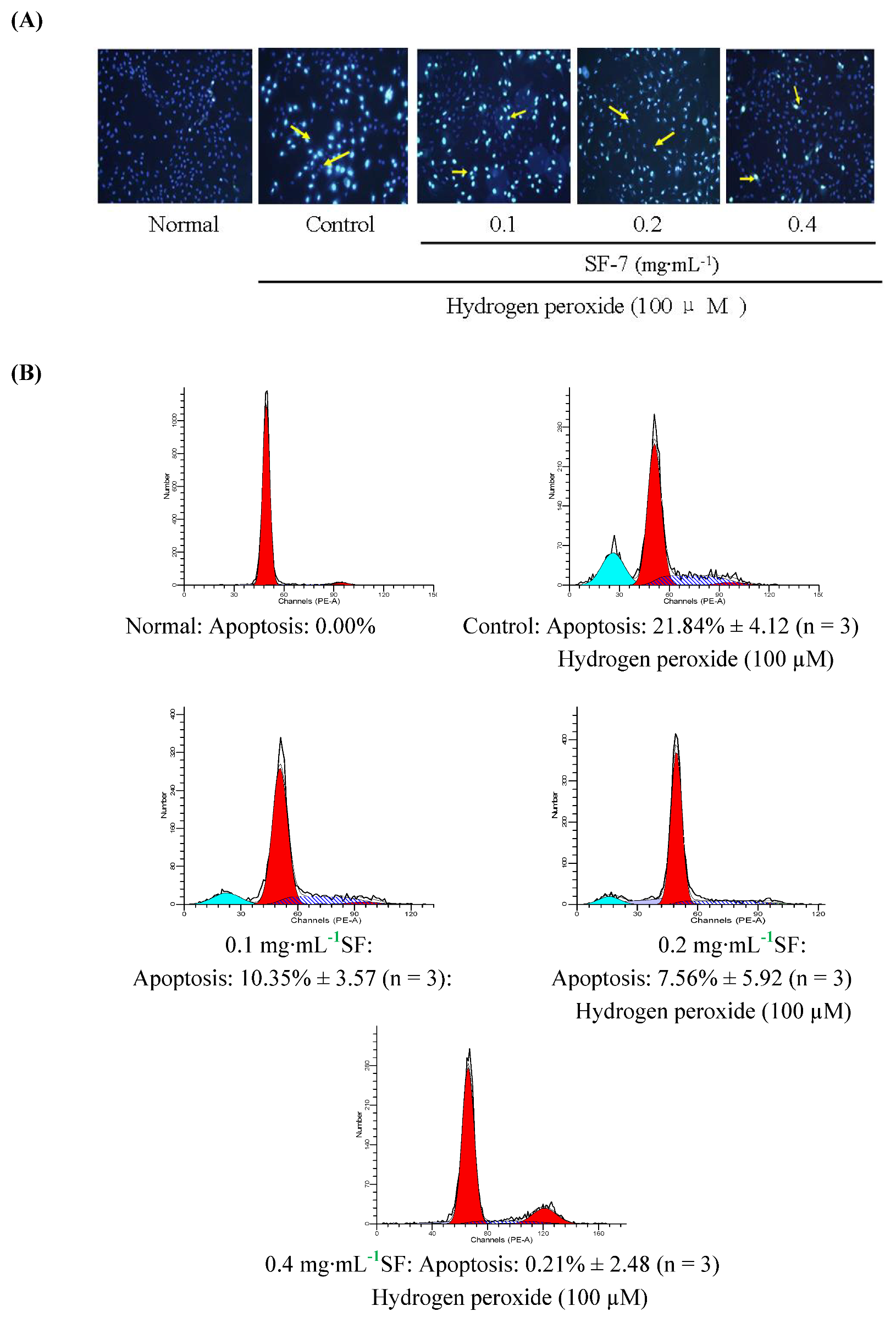
2.4. Effect of SF-7 on intracellular ROS concentration in H2O2-injured VSMCs

2.5. Effect of SF-7 on intracellular Ca2+ concentration in H2O2-injured VSMCs

3. Experimental
3.1. Materials
3.2. Preparation for active fraction SF-7

3.3. Cell culture and drug treatment
3.4. Assay of cell viability with the MTT method
3.5. Detection of LDH leakage, intracellular MDA level and SOD activity with spectrophotometry
3.6. Observation of VSMCs apoptosis by nuclear staining with Hoechst33342
3.7. Detection of apoptotic cells
3.8. Assay for intracellular ROS
3.9. Determination of intracellular Ca2+ concentration
3.10. Statistical analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds. are available from the authors.
References
- Zhang, J.; Sun, C.L. Application in gynecology diseases of Shaofu Zhuyu decoction. Chin. J. Nat. Med. 2002, 4, 109–112. [Google Scholar]
- Ye, X.L.; Wang, H.; Le, J.; Chen, X. Effects of Shaofu Zhuyu decoction on uterine convulsion and anti-inflammatory action in rodent. Chin. J. Hosp. Pharm. 2002, 22, 329–332. [Google Scholar]
- Su, S.L.; Duan, J.A. Wang, T.J. Yu; Hua, Y.Q.; Tang, Y.P. Evaluating the Effects of Shaofu Zhuyu Decoction on Hemorheology and Ovarian Function in Rat Model of Han-Ning Blood Stasis. Chin. J. Exp. Trad. Med. Form 2008, 14, 41–43. [Google Scholar]
- Hua, Y.Q.; Duan, J.A.; Zhu, Q.; Wang, Q.J. Study on method of oxytocin induced in vitro dysmenorrhea model in mouse. Chin. Pharmacol. Bull. 2007, 24, 489–493. [Google Scholar]
- Su, S.L.; Hua, Y.Q.; Duan, J.A.; Tang, Y.P.; Lu, Y.; Ding, A.W. In vitro inhibition on the contraction of isolated mouse uterine and chemical components of Shaofu Zhuyu Decoction. J. China Pharm. Univ. 2007, 38, 544–548. [Google Scholar]
- Hool, L.C.; Corry, B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid. Redox Sign 2007, 9, 409–435. [Google Scholar] [CrossRef]
- Heistad, D.D. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 689–695. [Google Scholar]
- Zhang, Z.; Wei, T.; Hou, J.; Li, G.; Yu, S.; Xin, W. Iron-induced oxidative damage and apoptosis in cerebellar granule cells: attenuation by tetramethylpyrazine and ferulic acid. Eur. J. Pharmacol. 2003, 467, 41–47. [Google Scholar] [CrossRef]
- Wu, S.J.; Ng, L.T.; Lin, C.C. Antioxidant activities of some common ingredients of traditional Chinese medicine, Angelica sinensis, Lycium barbarum and Poria cocos. Phytother. Res. 2004, 18, 1008–1012. [Google Scholar]
- Hou, Y.Z.; Zhao, G.R.; Yang, J.; Yuan, Y.J.; Zhu, G.G.; Hiltunen, R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci. 2004, 75, 1775–1786. [Google Scholar] [CrossRef]
- Mak, D.H.; Chiu, P.Y.; Dong, T.T.; Tsim, K.W.; Ko, K.M. Dang-Gui Bu Xue Tang produces a more potent cardioprotective effect than its component herb extracts and enhances glutathione status in rat heart mitochondria and erythrocytes. Phytother. Res. 2006, 20, 561–567. [Google Scholar] [CrossRef]
- Kuang, X.; Yao, Y.; Du, J.R.; Liu, Y.X.; Wang, C.Y.; Qian, Z.M. Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Res. 2006, 1102, 45–53. [Google Scholar]
- Yang, X.; Zhao, Y.; Lv, Y.; Yang, Y.; Ruan, Y. Protective effect of polysaccharide fractions from Radix A. sinensis against tert-butylhydroperoxide induced oxidative injury in murine peritoneal macrophages. J. Biochem. Mol. Biol. 2007, 40, 928–935. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Zhou, Y.; Lv, Y.; Mao, J.; Zhao, P. Component and antioxidant properties of polysaccharide fractions isolated from Angelica sinensis (OLIV.) DIELS. Biol. Pharm. Bull. 2007, 30, 1884–90. [Google Scholar] [CrossRef]
- Yu, Y.; Du, J.R.; Wang, C.Y.; Qian, Z.M. Protection against hydrogen peroxide-induced injury by Z-ligustilide in PC12 cells. Exp. Brain Res. 2008, 184, 307–312. [Google Scholar]
- Su, S.L.; Yu, L.; Hua, Y.Q.; Duan, J.A.; Deng, H.S.; Tang, Y.P.; Lu, Y.; Ding, A.W. Screening and analyzing the potential bioactive components from Shaofu Zhuyu decoction, using human umbilical vein endothelial cell extraction and high-performance liquid chromatography coupled with mass spectrometry. Biomed. Chromatogr. 2008, 22, 1385–1392. [Google Scholar] [CrossRef]
- Li, Y.J.; Guan, H.D. Effects of alpinetin on rat vascular smooth muscle cells. J. Asian Nat. Prod. Res. 2004, 6, 87–92. [Google Scholar]
- Li, P.F.; Dietz, R.; Harsdorf, R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation 1997, 96, 3602–3609. [Google Scholar] [CrossRef]
- Guo, F.; Zhu, B.Y.; Chi, X.L.; Huang, H.L. Inhibition of rosmarinic acid on the apoptosis of vascular smooth muscle cells induced by hydrogen peroxide. Chin. Pharmacol. Bull. 2007, 23, 365–369. [Google Scholar]
- Wu, L.; Li, X.R.; Li, Y.H.; Wang, L.J.; Tang, Y.; Xue, M. Proliferative inhibition of danxiongfang and its active ingredients on rat vascular smooth muscle cell and protective effect on the VSMC damage induced by hydrogen peroxide. J. Ethnopharmacol. 2009, 126, 197–206. [Google Scholar]
- Xiao, X.H.; Liu, J.T.; Hu, J.W.; Zhu, X.P.; Yang, H.; Wang, C.Y.; Zhang, Y.H. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca2+ antagonism and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 591, 21–27. [Google Scholar] [CrossRef]
- Satpute, R.M.; Kashyap, R.S.; Deopujari, J.Y.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Protection of PC12 cells from chemical ischemia induced oxidative stress by Fagonia Arabica. Food Chem. Toxicol. 2009, 47, 2689–2695. [Google Scholar] [CrossRef]
- Kwok, H.H.; Ng, W.Y.; Yang, M.S.M.; Mak, N.K.; Wong, R.N.S.; Yue, P.Y.K. The ginsenoside protopanaxatriol protects endothelial cells from hydrogen peroxide-induced cell injury and cell death by modulating intracellular redox status. Free Radical Biol. Med. 2010, 48, 437–445. [Google Scholar]
- Hearse, D.J.; Maxwell, L.; Saldanha, C.; Gavin, J.B. The myocardial vasculature during ischemia and reperfusion: a target for injury and protection. J. Mol. Cell. Cardiol. 1993, 25, 759–800. [Google Scholar] [CrossRef]
- Berliner, J.A.; Navab, M.; Fogelman, A.M.; Frank, J.S.; Demer, L.L.; Edwards, P.A.; Waston, A.D.; Lusis, A.J. Atherosclerosis: basic mechanisms, oxidation, inflammation, and genetics. Circulation 1995, 91, 2488–2496. [Google Scholar] [CrossRef]
- Heo, S.K.; Yi, H.S.; Yun, H.J.; Ko, C.H.; Choi, J.W.; Park, S.D. Ethylacetate extract from Draconis Resina inhibits LPS-induced inflammatory responses in vascular smooth muscle cells and macrophages via suppression of ROS production. Food Chem. Toxicol. 2010, 48, 1129–1136. [Google Scholar]
- Kojima, K.; Kume, H.; Ito, S.; Oguma, T.; Shiraki, A.; Kondo, M.; Ito, Y.; Shimokata, K. Direct effects of hydrogen peroxide on airway smooth muscle tone: Roles of Ca2+ influx and Rho-kinase. Eur. J. Pharmacol. 2001, 556, 151–156. [Google Scholar]
- Zhang, L.; Yu, H.X.; Sun, Y.; Lin, X.F.; Chen, B.; Chen, T.; Cao, G.X.; Wang, Z.W. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2007, 564, 18–25. [Google Scholar] [CrossRef]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar]
- Li, C.R.; Zhou, Z.; Zhu, D.; Sun, Y.N.; Dai, J.M.; Wang, S.Q. Protective effect of paeoniflorin on irradiation-induced cell damage involved in modulation of reactive oxygen species and the mitogen-activated protein kinases. Int. J. Biochem. Cell Biol. 2007, 39, 426–438. [Google Scholar] [CrossRef]
- Kim, S.H.; Kumar, C.N.; Kim, H.J.; Kim, D.H.; Cho, J.; Jin, C.; Lee, Y.S. Glucose-containing flavones—their synthesis and antioxidant and neuroprotective activities. Bioorg. Med. Chem. Lett. 2009, 19, 6009–6013. [Google Scholar]
- Bao, X.J.; Su, S.L.; Duan, J.A.; Hua, Y.Q.; Tang, Y.P.; Shang, E.X. Simultaneous analyze chemical components of inhibiting mice uterine contraction in active fraction of Shaofu Zhuyu Decoction. Chin. J. Exp. Trad. Med. Form 2008, 14, 38–41. [Google Scholar]
- Zhang, H.J.; Shen, P.; Cheng, Y.Y. Identification and determination of the major constituents in traditional Chinese medicine Si-Wu-Tang by HPLC coupled with DAD and ESI-MS. J. Pharm. Biomed. Anal. 2004, 34, 705–713. [Google Scholar] [CrossRef]
- Liang, Q.M.; Yu, X.F.; Qu, S.C.; Xu, H.L.; Sui, D.Y. Acanthopanax senticosides B ameliorates oxidative damage induced by hydrogen peroxide in cultured neonatal rat cardiomyocytes. Eur. J. Pharmacol. 2010, 627, 209–215. [Google Scholar]
- Liu, H.T.; Li, W.M.; Xu, G.; Li, X.Y.; Bai, X.F.; Wei, P.; Yu, C.; Du, Y.G. Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol. Res. 2009, 59, 167–175. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, L.; Duan, J.A.; Tang, Y.P.; Ma, H.Y.; Su, S.L.; Guo, J.M.; Hua, Y.Q. The Protective Effects of the Active Fraction of Shaofu Zhuyu Decoction on Hydrogen Peroxide-Induced Oxidative Injury in Vascular Smooth Muscle Cells. Molecules 2010, 15, 5066-5078. https://doi.org/10.3390/molecules15085066
Liu L, Duan JA, Tang YP, Ma HY, Su SL, Guo JM, Hua YQ. The Protective Effects of the Active Fraction of Shaofu Zhuyu Decoction on Hydrogen Peroxide-Induced Oxidative Injury in Vascular Smooth Muscle Cells. Molecules. 2010; 15(8):5066-5078. https://doi.org/10.3390/molecules15085066
Chicago/Turabian StyleLiu, Li, Jin Ao Duan, Yu Ping Tang, Hong Yue Ma, Shu Lan Su, Jian Ming Guo, and Yong Qing Hua. 2010. "The Protective Effects of the Active Fraction of Shaofu Zhuyu Decoction on Hydrogen Peroxide-Induced Oxidative Injury in Vascular Smooth Muscle Cells" Molecules 15, no. 8: 5066-5078. https://doi.org/10.3390/molecules15085066




