Catalytic Conversion of Cellulose to Levulinic Acid by Metal Chlorides
Abstract
:1. Introduction

2. Results and Discussion
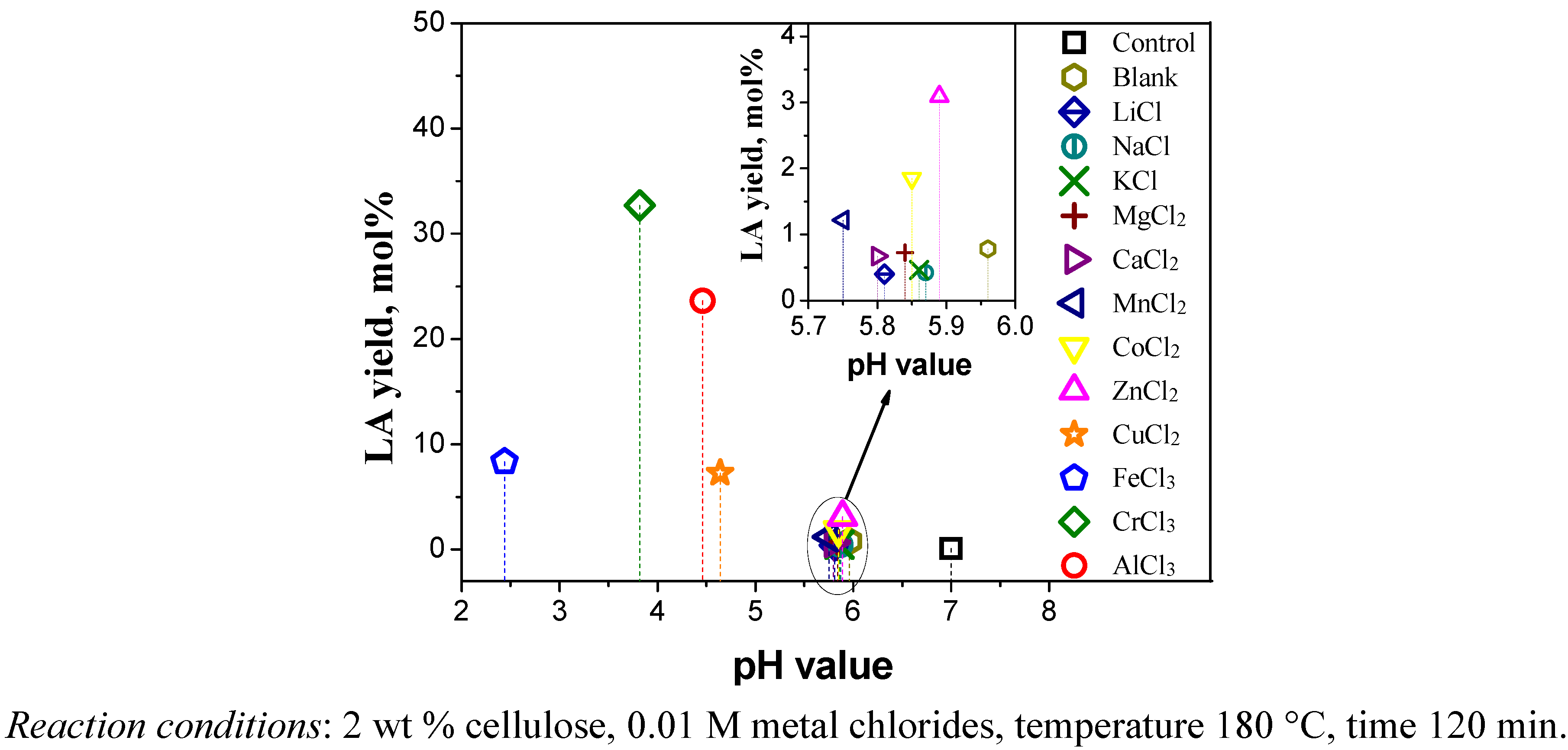
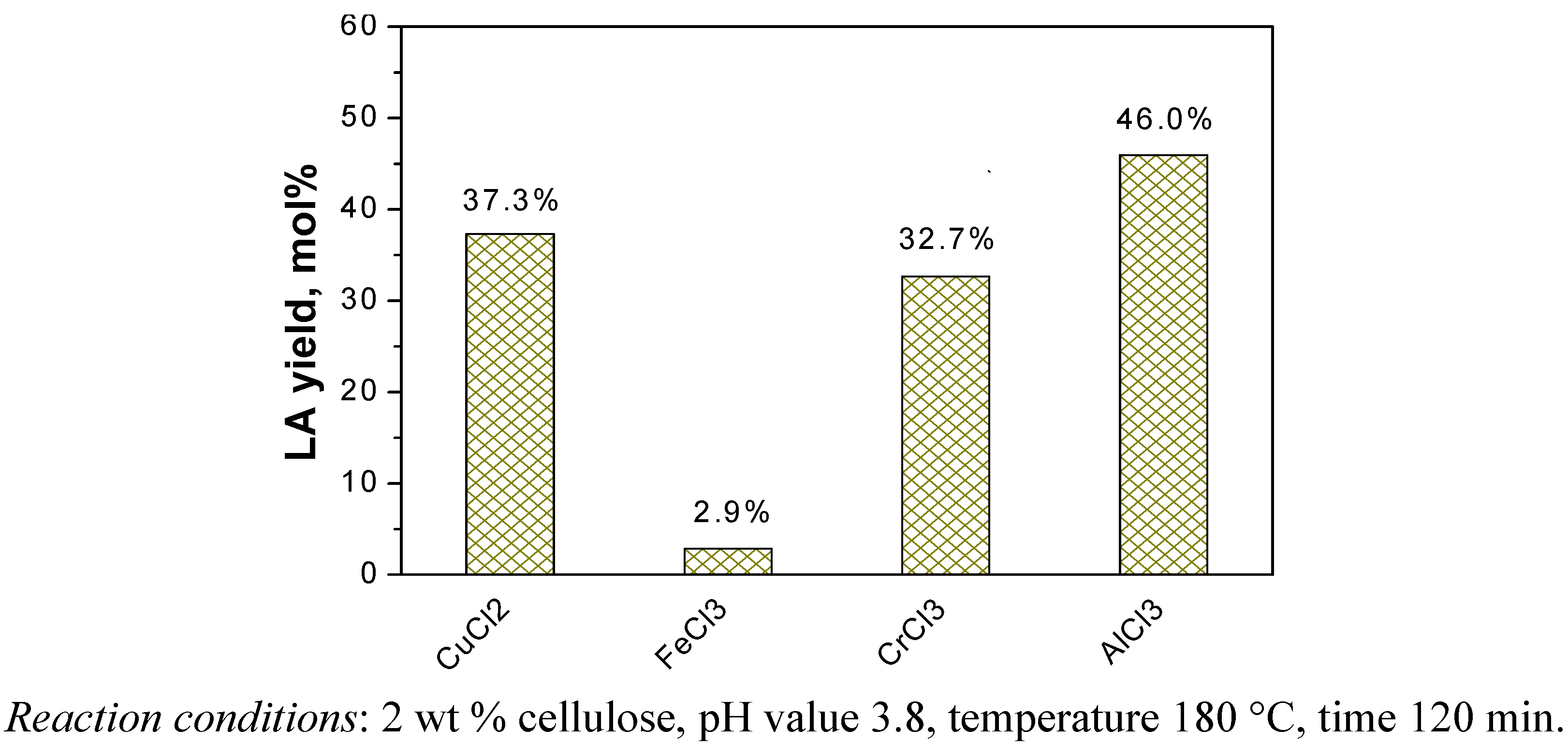
2.1. Catalytic Effects of Metal Chlorides on the Conversion of Cellulose


2.2. Relationship between Reactivity and Acidity of Reaction System
2.3. Effects of Reaction Conditions on Yields of Products
2.3.1. Agitation Speed

2.3.2. Reaction Time

2.3.3. Reaction Temperature

2.3.4. Catalyst Dosage

2.3.5. Substrate Concentration

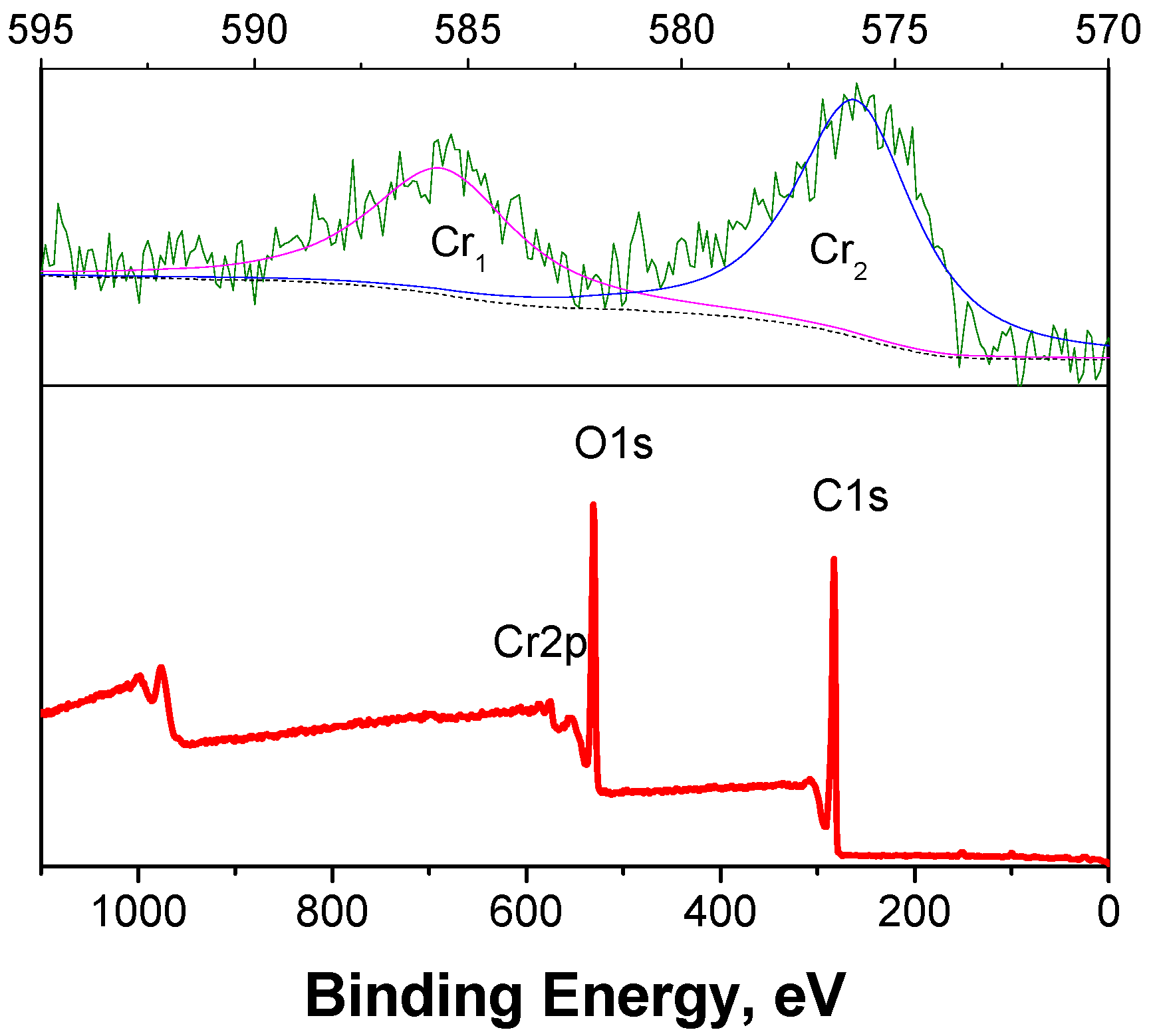
2.4. Metal Tracing and Recycling

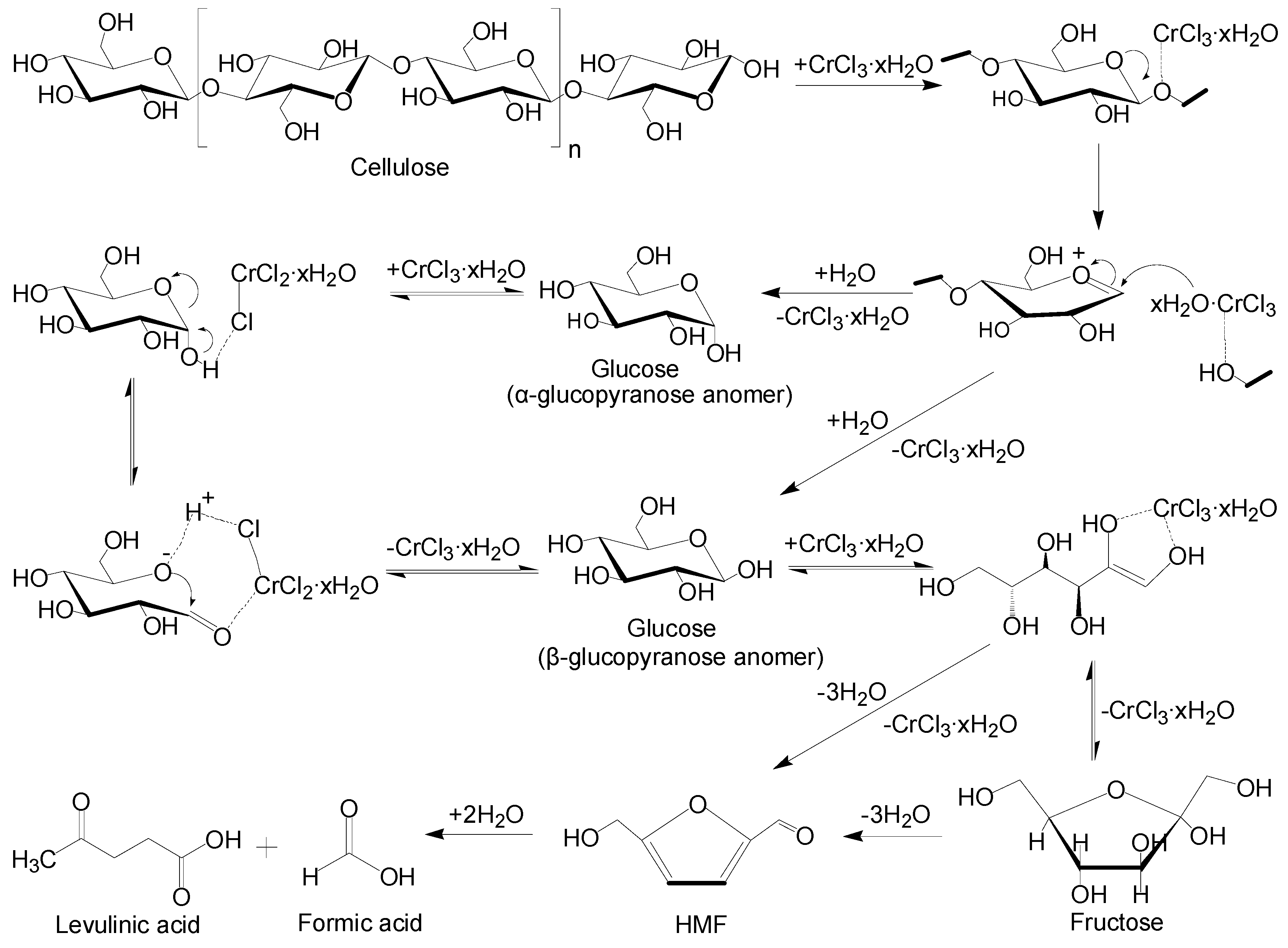
2.5. Mechanism of Catalytic Degradation
3. Experimental
3.1. Materials
3.2. Equipment and Procedure
3.3. Sample Analysis
4. Conclusions
Acknowledgements
References
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Girisuta, B.; Danon, B.; Manurung, R.; Janssen, L.P.; Heeres, H.J. Experimental and kinetic modelling studies on the acid-catalysed hydrolysis of the water hyacinth plant to levulinic acid. Bioresour. Technol. 2008, 99, 8367–8375. [Google Scholar]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recy. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Efremov, A.A.; Pervyshina, G.G.; Kuznetsov, B.N. Production of levulinic acid from wood raw material in the presence of sulfuric acid and its salts. Chem. Nat. Compd. 1998, 34, 182–185. [Google Scholar] [CrossRef]
- Chang, C.; Cen, P.L.; Ma, X.J. Levulinic acid production from wheat straw. Bioresour. Technol. 2007, 98, 1448–1453. [Google Scholar] [CrossRef]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. Kinetic study on the acid-catalyzed hydrolysis of cellulose to levulinic acid. Ind. Eng. Chem. Res. 2007, 46, 1696–1708. [Google Scholar] [CrossRef]
- Yan, L.F.; Yang, N.K.; Pang, H.; Liao, B. Production of levulinic acid from bagasse and paddy straw by liquefaction in the presence of hydrochloride acid. Clean-Soil, Air, Water 2008, 36, 158–163. [Google Scholar] [CrossRef]
- Lange, J.P.; van de Graaf, W.D.; Haan, R.J. Conversion of furfuryl alcohol into ethyl levulinate using solid acid catalysts. Chem. Sus. Chem. 2009, 2, 437–441. [Google Scholar]
- Fan, G.D.; Shen, M.; Zhang, Z.; Jia, F. Preparation, characterization and catalytic properties of S2O82-/ZrO2-CeO2 solid superacid catalyst. J. Rare Earth. 2009, 27, 437–442. [Google Scholar] [CrossRef]
- Wang, P.; Zhan, S.H.; YU, H.B. Production of levulinic acid from cellulose catalyzed by environmental-friendly catalyst. Adv. Mater. Res. 2010, 96, 183–187. [Google Scholar]
- Seri, K.I.; Sakski, T.; Shibata, M.; Inoue, Y., Ishida. Lanthanum (III)-catalyzed degradation of cellulose at 250 °C. Bioresour. Technol. 2002, 81, 257–260. [Google Scholar] [CrossRef]
- Zhao, H.B.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar]
- Li, C.Z.; Zhang, Z.H.; Zhao, Z.B.K. Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Lett. 2009, 50, 5403–5405. [Google Scholar] [CrossRef]
- Rasrendra, C.B.; Makertihartha, I.G.B.N.; Adisasmito, S.; Heeres, H.J. Green chemicals from D-glucose: Systematic studies on catalytic effects of inorganic salts on the chemo-selectivity and yield in aqueous solutions. Topic Catalysis 2010, 53, 1241–1247. [Google Scholar]
- Lu, C.B.; Lü, X.Y. Effect of metal chlorides on glucose decomposition kinetics in high temperature liquid water. CIESC J. 2009, 60, 3035–3041. [Google Scholar]
- Bicker, M.; Endres, S.; Ott, L.; Vogel, H. Catalytical conversion of carbohydrates in subcritical water: A new chemical process for lactic acid production. J. Mol. Catal. A: Chem. 2005, 239, 151–157. [Google Scholar] [CrossRef]
- Kong, L.Z.; Li, G.M.; Wang, H.; He, W.Z.; Ling, F. Hydrothermal catalytic conversion of biomass for lactic acid production. J. Chem. Technol. Biotechnol. 2008, 83, 383–388. [Google Scholar] [CrossRef]
- Kuster, B.F.M.; van der Baan, H.S. Dehydration of D-fructose (formation of 5-hydroxymethyl-2-furaldehyde and levulinic acid).2.Influence of initial and catalyst concentrations on dehydration of D-fructose. Carbohydr. Res. 1977, 54, 165–176. [Google Scholar] [CrossRef]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. Green chemicals: A kinetic study on the conversion of glucose to Levulinic acid. Chem. Eng. Res. Des. 2006, 84, 339–349. [Google Scholar] [CrossRef]
- Devulapelli, V.G.; Weng, H.S. Synthesis of cinnamyl acetate by solid-liquid phase transfer catalysis: Kinetic study with a batch reactor. Catal. Commun. 2009, 10, 1638–1642. [Google Scholar] [CrossRef]
- Mckibbins, S.; Harris, J.F.; Saeman, J.H.; Neill, W.K. Kinetics of the acid catalyzed conversion of glucose to 5-hydroxymethyl-2-furaldehyde and levulinic acid. Forest Prod. J. 1962, 12, 17–23. [Google Scholar]
- Amarasekara, A.S.; Ebede, C.C. Zinc chloride mediated degradation of cellulose at 200 °C and identification of the products. Bioresour. Technol. 2009, 100, 5301–5304. [Google Scholar] [CrossRef]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J. Am. Chem. Soc. 2008, 130, 12787–12793. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng, L.; Lin, L.; Zhang, J.; Zhuang, J.; Zhang, B.; Gong, Y. Catalytic Conversion of Cellulose to Levulinic Acid by Metal Chlorides. Molecules 2010, 15, 5258-5272. https://doi.org/10.3390/molecules15085258
Peng L, Lin L, Zhang J, Zhuang J, Zhang B, Gong Y. Catalytic Conversion of Cellulose to Levulinic Acid by Metal Chlorides. Molecules. 2010; 15(8):5258-5272. https://doi.org/10.3390/molecules15085258
Chicago/Turabian StylePeng, Lincai, Lu Lin, Junhua Zhang, Junping Zhuang, Beixiao Zhang, and Yan Gong. 2010. "Catalytic Conversion of Cellulose to Levulinic Acid by Metal Chlorides" Molecules 15, no. 8: 5258-5272. https://doi.org/10.3390/molecules15085258



