Neuroscientists as Cartographers: Mapping the Crossroads of Gonadal Hormones, Memory and Age Using Animal Models
Abstract
:- 1.
- Why the study of cognitive aging is important: An introduction
- 2.
- Gonadal hormones, spatial cognition, and aging: A tertiary model
- 3.
- Aging and spatial cognition: A gonadal hormone perspective
- 3.1.
- Menopause
- 3.2.
- Age-related changes in spatial cognition
- 3.3.
- Specific “internal secretions” as mediators of various cognitive functions
- 3.4.
- Rodents as a clinically relevant model to test hormone loss and treatment
- 3.5.
- Impact of estrogens and progesterone on cognition
- 3.5.1.
- Ovarian hormone loss and cognition
- 3.5.2.
- Estrogens and cognition
- 3.5.3.
- Progestins and cognition
- 3.6.
- Impact of androgens on cognition
- 3.7.
- Impact of gonadotropins on cognition
- 4.
- Gonadal hormones, spatial cognition and aging: Postulated brain mechanisms
- 4.1.
- Synaptic plasticity in cognitive brain regions
- 4.2.
- Basal forebrain cholinergic neurons
- 4.3.
- Genomic and non-genomic actions
- 4.
- Neuroprotective and neurotrophic effects
- 5.
- Scopes of research within the tertiary model: Prior journeys, future directions
- 6.
- A final comment: How understanding the crossroads of gonadal hormones, memory, and age can help draw a map to optimal brain aging
1. Why the Study of Cognitive Aging Is Important: An Introduction
2. Gonadal Hormones, Spatial Cognition, and Aging: A Tertiary Model
3. Aging and Spatial Cognition: A Gonadal Hormone Perspective
3.1. Menopause

3.2. Age-related changes in spatial cognition
3.3. Specific “internal secretions” as mediators of various cognitive functions
3.4. Rodents as a clinically relevant model to test hormone loss and treatment
3.5. Impact of estrogens and progesterone on cognition
3.5.1. Ovarian hormone loss and cognition
3.5.2. Estrogens and cognition
3.5.3. Progestins and cognition
3.6. Impact of androgens on cognition
3.7. Impact of gonadotropins on cognition
4. Gonadal Hormones, Spatial Cognition and Aging: Postulated Brain Mechanisms
4.1. Synaptic plasticity in cognitive brain regions
4.2. Basal forebrain cholinergic neurons
4.3. Genomic and non-genomic actions
4.4. Neuroprotective and neurotrophic effects
5. Scopes of Research within the Tertiary Model: Prior Journeys, Future Directions
6. A Final Comment: How Understanding the Crossroads of Gonadal Hormones, Memory, and Age Can Help Draw a Map to Optimal Brain Aging
Acknowledgements
References and Notes
- U.S. Census Bureau. Population Information. http://www.census.gov/ipc/www/world.html/. (accessed online on April 21, 2007.).
- Erickson, C.A.; Barnes, C.A. The neurobiology of memory changes in normal aging. Exp. Gerontol. 2003, 38, 61–69. [Google Scholar] [CrossRef]
- Tulving, E.; Craik, F.I. The Oxford Handbook of Memory; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Kausler, D.H. Learning and Memory in Normal Aging; Academic Press: New York, NY, USA, 1994. [Google Scholar]
- Balota, D.A.; Dolan, P.O.; Duchek, J.M. Memory changes in healthy young and older adults. In The Oxford Handbook of Memory; Oxford University Press: New York, NY, USA, 2000; pp. 395–410. [Google Scholar]
- Albert, M.; Duffy, F.H.; Naeser, M. Nonlinear changes in cognition with age and their neuropsychologic correlates. Can. J. Psychol. 1987, 41, 141–157. [Google Scholar] [PubMed]
- Albert, M.S. Memory decline: the boundary between aging and age-related disease. Annal. Neurol. 2002, 51, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.A. Memory changes during normal aging: Neurobiological correlates. In Neurobiology of Learning and Memory; Martinez, J.A., Jr., Kesner, R.P., Eds.; Academic Press: San Diego, CA, USA, 1998; pp. 247–273. [Google Scholar]
- Tolman, E.C. Cognitive maps in rats and men. Psychol. Rev. 1948, 55, 189–208. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Clarendon Press: Oxford, England, 1978. [Google Scholar]
- Eichenbaum, H. A cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci. 2000, 1, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.N.; Barnes, C.A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Kabaso, D.; Coskren, P.J.; Henry, B.I.; Hof, P.R.; Wearne, S.L. The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging. Cereb. Cortex 2009, 19, 2248–2268. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A.D.; Hitch, G.J. Working Memory. In The Psychology of Learning and Motivation: Advances in Research and Theory; Bower, G.A., Ed.; Academic Press: New York, NY, USA, 1974; Volume 8, pp. 47–90. [Google Scholar]
- Olton, D.S.; Becker, J.T.; Handelmann, G.E. Hippocampus, space, and memory. Behav. Brain Sci. 1979, 2, 313–365. [Google Scholar] [CrossRef]
- Armstrong, D.M.; Sheffield, R.; Buzsaki, G.; Chen, K.S.; Hersh, L.B.; Nearing, B.; Gage, F.H. Morphologic alterations of choline acetyltransferase-positive neurons in the basal forebrain of aged behaviorally characterized Fisher 344 rats. Neurobiol. Aging 1993, 14, 457–470. [Google Scholar] [CrossRef]
- Croll, S.D.; Ip, N.Y.; Lindsay, R.M.; Wiegand, S.J. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998, 812, 200–208. [Google Scholar] [CrossRef]
- Diana, G.; Domenici, M.R.; Scotti de Carolis, A.; Loizzo, A.; Sagratella, S. Reduced hippocampal CA1 Ca(2+)-induced long-term potentiation is associated with age-dependent impairment of spatial learning. Brain Res. 1995, 686, 107–110. [Google Scholar] [CrossRef]
- Fischer, W.; Bjorklund, A.; Chen, K.; Gage, F.H. NGF improves spatial memory in aged rodents as a function of age. J. Neurosci. 1991, 11, 1889–1906. [Google Scholar] [CrossRef] [PubMed]
- Hasenohrl, R.U.; Soderstrom, S.; Mohammed, A.H.; Ebendal, T.; Huston, J.P. Reciprocal changes in expression of mRNA for nerve growth factor and its receptors TrkA and LNGFR in brain of aged rats in relation to maze learning deficits. Exp. Brain Res. 1997, 114, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.D.; Kearns, C.E.; Winn, S.R.; Frydel, B.; Emerich, D.F. Effects of intraventricular encapsulated hNGF-secreting fibroblasts in aged rats. Cell Transplant. 1996, 5, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.G.; Gage, F.H. Anticholinergic sensitivity in the aging rat septohippocampal system as assessed in a spatial memory task. Neurobiol. Aging 1993, 14, 487–497. [Google Scholar] [CrossRef]
- Quirion, R.; Wilson, A.; Rowe, W.; Aubert, I.; Richard, J.; Doods, H.; Parent, A.; White, N.; Meaney, M.J. Facilitation of acetylcholine release and cognitive performance by an M(2)-muscarinic receptor antagonist in aged memory-impaired. J. Neurosci. 1995, 15, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Rapp, P.R.; Gallagher, M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Nat. Acad. Sci. USA 1996, 93, 9926–9930. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, K.; Greene, R.; Personett, D.; Robbins, M.; Kent, C.; Bryan, D.; Skiba, E.; Gallagher, M.; McKinney, M. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiol. Aging 1998, 19, 351–361. [Google Scholar] [CrossRef]
- Croll, S.D.; Chesnutt, C.R.; Greene, N.A.; Lindsay, R.M.; Wiegand, S.J. Peptide immunoreactivity in aged rat cortex and hippocampus as a function of memory and BDNF infusion. Pharmacol. Biochem. Behav. 1999, 64, 625–635. [Google Scholar] [CrossRef]
- Timaras, P.; Quay, W.; Vernadakis, A. Hormones and Aging; CRC Press: New York, NY, USA, 1995. [Google Scholar]
- Downs, J.L.; Wise, P.M. The role of the brain in female reproductive aging. Mol. Cell. Endocrinol. 2009, 299, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Meites, J.; Lu, J.K.H. Reproductive aging and neuroendocrine function. In Oxford Review of Reproductive Biology; Charlton, H.M., Ed.; Oxford Press: New York, NY, USA, 1994. [Google Scholar]
- Newman, M.; Kasznaik, A. Spatial memory and aging: performance on a human analog of the Morris water maze. Aging, Neuropsychol. Cogn. 2000, 7, 86–93. [Google Scholar] [CrossRef]
- Wilkniss, S.M.; Jones, M.G.; Korol, D.L.; Gold, P.E.; Manning, C.A. Age-related differences in an ecologically based study of route learning. Psychol. Aging 1997, 12, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Rapp, P.R.; Kansky, M.T.; Roberts, J.A. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport 1997, 8, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Head, E.; Mehta, R.; Hartley, J.; Kameka, M.; Cummings, B.J.; Cotman, C.W.; Ruehl, W.W.; Milgram, N.W. Spatial learning and memory as a function of age in the dog. Behav. Neurosci 1995, 109, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.A.; Nadel, L.; Honig, W.K. Spatial memory deficit in senescent rats. Can. J. Psychol. 1980, 34, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, H.A.; Nelson, M.E.; Granholm, A.C. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol. Aging 2003, 24, 37–48. [Google Scholar] [CrossRef]
- Markowska, A.L. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J. Neurosci. 1999, 19, 8122–8133. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Sanberg, P.R.; Sengstock, G.J. Nicotine enhances the learning and memory of aged rats. Pharmacol. Biochem. Behav. 1995, 52, 517–523. [Google Scholar] [PubMed]
- Beatty, W.W.; Bierley, R.A.; Boyd, J.G. Preservation of accurate spatial memory in aged rats. Neurobiol. Aging 1985, 6, 219–225. [Google Scholar] [CrossRef]
- Bond, N.W.; Everitt, A.V.; Walton, J. Effects of dietary restriction on radial-arm maze performance and flavor memory in aged rats. Neurobiol. Aging 1989, 10, 27–30. [Google Scholar] [CrossRef]
- Chrobak, J.J.; Hanin, I.; Lorens, S.A.; Napier, T.C. Within-subject decline in delayed-non-match-to-sample radial arm maze performance in aging Sprague-Dawley rats. Behav. Neurosci. 1995, 109, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.M.; Baxter, M.G.; Markowska, A.L.; Olton, D.S.; Price, D.L. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol. Aging 1995, 16, 149–160. [Google Scholar] [CrossRef]
- Kadar, T.; Arbel, I.; Silbermann, M.; Levy, A. Morphological hippocampal changes during normal aging and their relation to cognitive deterioration. J. Neural Transm. 1994, 44, 133–143. [Google Scholar]
- Lebrun, C.; Durkin, T.P.; Marighetto, A.; Jaffard, R. A comparison of the working memory performances of young and aged mice combined with parallel measures of testing and drug-induced activations of septo-hippocampal and nbm-cortical cholingergic neurones. Neurobiol. Aging 1990, 11, 515–521. [Google Scholar] [CrossRef]
- Lindner, M.D.; Balch, A.H.; VanderMaelen, C.P. Short forms of the "reference-" and "working-memory" Morris water maze for assessing age-related deficits. Behav. Neural Biol. 1992, 58, 94–102. [Google Scholar] [CrossRef]
- Noda, Y.; Yamada, K.; Nabeshima, T. Role of nitric oxide in the effect of aging on spatial memory in rats. Behav. Brain Res. 1997, 83, 153–158. [Google Scholar] [CrossRef]
- Pitsikas, N.; Algeri, S. Deterioration of spatial and nonspatial reference and working memory in aged rats: Protective effect of life-long calorie restriction. Neurobiol. Aging 1992, 13, 369–373. [Google Scholar] [CrossRef]
- Rapp, P.R.; Rosenberg, R.A.; Gallagher, M. An evaluation of spatial information processing in aged rats. Behav. Neurosci. 1987, 101, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; McEwen, J.J.; Szrengiel, A.; Joseph, J.A. Effect of age on the radial arm water maze-a test of spatial learning and memory. Neurobiol. Aging 2004, 25, 223–229. [Google Scholar] [CrossRef]
- Stewart, J.; Mitchell, J.; Kalant, N. The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazes. Neurobiol. Aging 1989, 10, 669–675. [Google Scholar] [CrossRef]
- Wallace, J.E.; Krauter, E.E.; Campbell, B.A. Animal models of declining memory in the aged: short-term and spatial memory in the aged rat. J. Gerontol. 1980, 35, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Wellman, C.L.; Pelleymounter, M.A. Differential effects of nucleus basalis lesions in young adult and aging rats. Neurobiol. Aging 1999, 20, 381–393. [Google Scholar] [CrossRef]
- Wyss, J.M.; Chambless, B.D.; Kadish, I.; van Groen, T. Age-related decline in water maze learning and memory in rats: strain differences. Neurobiol. Aging 2000, 21, 671–681. [Google Scholar] [CrossRef]
- Talboom, J.S.; Williams, B.J.; Baxley, E.R.; West, S.G.; Bimonte-Nelson, H.A. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol. Learn. Memory 2008, 90, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, S. Behavioral impairment in radial-arm maze learning and acetylcholine content of the hippocampus and cerebral cortex in aged mice. Behav. Brain Res. 1994, 65, 103–111. [Google Scholar] [CrossRef]
- Salthouse, T.A.; Mitchell, D.R.; Palmon, R. Memory and age differences in spatial manipulation ability. Psychol. Aging 1989, 4, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Aggleton, J.P.; Blindt, H.S.; Candy, J.M. Working memory in aged rats. Behav. Neurosci. 1989, 103, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Bimonte-Nelson, H.A.; Singleton, R.S.; Hunter, C.L.; Price, K.L.; Moore, A.B.; Granholm, A.C. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav. Neurosci. 2003, 117, 1395–406. [Google Scholar] [CrossRef] [PubMed]
- Bimonte-Nelson, H.A.; Singleton, R.S.; Williams, B.J.; Granholm, A.C. Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav. Neurosci. 2004, 118, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Dunnett, S.B.; Evenden, J.L.; Iversen, S.D. Delay-dependent short-term memory deficits in aged rats. Psychopharmacology 1988, 96, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Winocur, G. Memory decline in aged rats: a neuropsychological interpretation. J. Gerontol. 1986, 41, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Zornetzer, S.F.; Thompson, R.; Rogers, J. Rapid forgetting in aged rats. Behav. Neural Biol. 1982, 36, 49–60. [Google Scholar] [CrossRef]
- Willig, F.; Palacios, A.; Monmaur, P.; M’Harzi, M.; Laurent, J.; Delacour, J. Short-term memory, exploration and locomotor activity in aged rats. Neurobiol. Aging 1987, 8, 393–402. [Google Scholar] [CrossRef]
- Adler, N.T. Neuroendocrinology of Reproduction: Physiology and Behavior; Plenum Press: New York, NY, USA, 1981. [Google Scholar]
- Beach, F.A. Hormones and mating behaviora in vertebrates. In Recent Progress in Hormone Research; Academic Press: New York, NY, USA, 1947. [Google Scholar]
- Luetters, C.; Huang, M.H.; Seeman, T.; Buckwalter, G.; Meyer, P.M.; Avis, N.E.; Sternfeld, B.; Johnston, J.M.; Greendale, G.A. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women’s health across the nation (SWAN). J. Women’s Health 2007, 16, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gil, E.; Canizares, S.; Torres, A.; de la Torre, F.; Halperin, I.; Salamero, M. Androgen treatment effects on memory in female-to-male transsexuals. Psychoneuroendocrinology 2009, 34, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Estrogen and cognitive aging in women. Neuroscience 2006, 138, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Sawaya, G.; Lieberburg, I.; Grady, D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. J. Am. Med. Assoc. 1998, 279, 688–695. [Google Scholar] [CrossRef]
- Asthana, S.; Craft, S.; Baker, L.D.; Raskind, M.A.; Birnbaum, R.S.; Lofgreen, C.P.; Veith, R.C.; Plymate, S.R. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology 1999, 24, 657–677. [Google Scholar] [CrossRef]
- Asthana, S.; Baker, L.D.; Craft, S.; Stanczyk, F.Z.; Veith, R.C.; Raskind, M.A.; Plymate, S.R. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology 2001, 57, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, T.; Isse, K.; Akazawa, K.; Hamamoto, M.; Yaoi, Y.; Hagino, N. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocr. J. 1994, 41, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, T.; Isse, K.; Akazawa, K.; Hamamoto, M.; Yaoi, Y.; Hagino, N. Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia 1995, 6, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Halbreich, U.; Lumley, L.A.; Palter, S.; Manning, C.; Gengo, F.; Joe, S.H. Possible acceleration of age effects on cognition following menopause. J. Psychiat. Res. 1995, 29, 153–163. [Google Scholar] [CrossRef]
- Sherwin, B.B.; Henry, J.F. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008, 29, 88–113. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Kochanek, K.D.; MacDorman, M.F. Advance report of final mortality statistics, 1994. Mon. Vital Stat. Rep. 1996, 45, 1–80. [Google Scholar]
- Amundsen, D.W.; Diers, C.J. The age of menopause in classical Greece and Rome. Hum. Biol. 1970, 42, 79–86. [Google Scholar] [PubMed]
- Amundsen, D.W.; Diers, C.J. The age of menopause in medieval Europe. Hum. Biol. 1973, 45, 605–612. [Google Scholar] [PubMed]
- Sherwin, B.B. Estrogen and cognitive functioning in women. Endocr. Rev. 2003, 24, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Estrogen and cognitive functioning in women. Proc. Soc. Exp. Biol. Med. 1998, 217, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L.; McIntosh, A.R.; Cabeza, R.; Habib, R.; Houle, S.; Tulving, E. General and specific brain regions involved in encoding and retrieval of events: what, where, and when. Proc. Nat. Acad. Sci. USA 1996, 93, 11280–11285. [Google Scholar] [CrossRef] [PubMed]
- Schacter, D.L.; Curran, T.; Reiman, E.M.; Chen, K.; Bandy, D.J.; Frost, J.T. Medial temporal lobe activation during episodic encoding and retrieval: a PET study. Hippocampus 1999, 9, 575–581. [Google Scholar] [CrossRef]
- Schacter, D.L.; Wagner, A.D. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 1999, 9, 7–24. [Google Scholar] [CrossRef]
- Grunwald, T.; Lehnertz, K.; Heinze, H.J.; Helmstaedter, C.; Elger, C.E. Verbal novelty detection within the human hippocampus proper. Proc. Nat. Acad. Sci. USA 1998, 95, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, T.; Kurthen, M. Novelty detection and encoding for declarative memory within the human hippocampus. Clin. EEG Neurosci. 2006, 37, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Resnick, S.M.; Maki, P.M.; Golski, S.; Kraut, M.A.; Zonderman, A.B. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm. Behav. 1998, 34, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.M.; Resnick, S.M. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol. Aging 2000, 21, 373–383. [Google Scholar] [CrossRef]
- Acosta, J.I.; Mayer, L.; Talboom, J.S.; Tsang, C.W.; Smith, C.J.; Enders, C.K.; Bimonte-Nelson, H.A. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology 2009, 150, 4248–4259. [Google Scholar] [CrossRef] [PubMed]
- Aenlle, K.K.; Kumar, A.; Cui, L.; Jackson, T.C.; Foster, T.C. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiol. Aging 2009, 30, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Pych, J.C.; Juraska, J.M. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm. Behav. 2002, 42, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Engler-Chiurazzi, E.; Tsang, C.; Nonnenmacher, S.; Liang, W.S.; Corneveaux, J.J.; Prokai, L.; Huentelman, M.J.; Bimonte-Nelson, H.A. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol. Aging 2010. In Press. [Google Scholar] [CrossRef] [PubMed]
- Braden, B.B.; Talboom, J.S.; Crain, I.D.; Simard, A.R.; Lukas, R.J.; Prokai, L.; Scheldrup, M.R.; Bowman, B.L.; Bimonte-Nelson, H.A. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol. Learn. Memory 2010, 93, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.I.; Mayer, L.; Braden, B.B.; Nonnenmacher, S.; Mennenga, S.; Bimonte, H.A. The cognitive effects of CEE depend on whether menopause is transitional or surgical. Endocrinology 2010, in press. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol. Aging 2000, 21, 107–116. [Google Scholar] [CrossRef]
- Frick, K.M.; Fernandez, S.M.; Bulinski, S.C. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience 2002, 115, 547–558. [Google Scholar] [CrossRef]
- Daniel, J.M.; Hulst, J.L.; Berbling, J.L. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 2006, 147, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.C.; Sharrow, K.M.; Kumar, A.; Masse, J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol. Aging 2003, 24, 839–852. [Google Scholar] [CrossRef]
- Bimonte-Nelson, H.A.; Francis, K.R.; Umphlet, C.D.; Granholm, A.C. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur. J. Neurosci. 2006, 24, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.L.; Savonenko, A.V. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen replacement withdrawal in aging rats. J. Neurosci. 2002, 22, 10985–10995. [Google Scholar] [CrossRef] [PubMed]
- Savonenko, A.V.; Markowska, A.L. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience 2003, 119, 821–830. [Google Scholar] [CrossRef]
- McDonald, R.J.; White, N.M. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 1993, 107, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G.; Garrud, P.; Rawlins, J.N.; O’Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 1982, 297, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992, 99, 195–231. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.M. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm. Behav. 2009, 55, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Wight, R.G.; Huang, M.H.; Avis, N.; Gold, E.B.; Joffe, H.; Seeman, T.; Vuge, M.; Karlamangla, A.S. Menopause-associated symptoms and cognitive performance: Results from the study of Women’s Health Across the Nation. Am. J. Epidemiol. 2010, 171, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Thilers, P.P.; Macdonald, S.W.; Nilsson, L.G.; Herlitz, A. Accelerated postmenopausal cognitive decline is restricted to women with normal BMI: longitudinal evidence from the Betula project. Psychoneuroendocrinology 2010, 35, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Mapstone, M. Memory complaints and memory performance in the menopausal transition. Menopause 2009, 16, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Farrag, A.K.; Khedr, E.M.; Abdel-Aleem, H.; Rageh, T.A. Effect of surgical menopause on cognitive functions. Dement. Geriat. Cogn. Disorder. 2002, 13, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Sherwin, B.B. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 1992, 17, 485–495. [Google Scholar] [CrossRef]
- Sherwin, B.B. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 1988, 13, 345–357. [Google Scholar] [CrossRef]
- Nappi, R.E.; Sinforiani, E.; Mauri, M.; Bono, G.; Polatti, F.; Nappi, G. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol. Obstet. Invest. 1999, 47, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, H.A.; Denenberg, V.H. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology 1999, 24, 161–173. [Google Scholar] [CrossRef]
- El-Bakri, N.K.; Islam, A.; Zhu, S.; Elhassan, A.; Mohammed, A.; Winblad, B.; Adem, A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J. Cell. Mol. Med. 2004, 8, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.M.; Roberts, S.L.; Dohanich, G.P. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol. Behav. 1999, 66, 11–20. [Google Scholar] [CrossRef]
- Feng, Z.; Cheng, Y.; Zhang, J.T. Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J. Pineal Res. 2004, 37, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B.; Johnson, D.A. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology 2008, 149, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Luine, V.; Arellanos, A.; Frankfurt, M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006, 1126, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Springer, L.N.; McAsey, M.E.; Flaws, J.A.; Tilly, J.L.; Sipes, I.G.; Hoyer, P.B. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol. Appl. Pharmacol. 1996, 139, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.P.; Devine, P.J.; Dyer, C.A.; Hoyer, P.B. The follicle-deplete mouse ovary produces androgen. Biol. Reprod. 2004, 71, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A.; Bower, J.H.; Maraganore, D.M.; Ahlskog, J.E.; Grossardt, B.R.; de Andrade, M.; Melton, L.J., 3rd. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007, 69, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8, 3–63. [Google Scholar] [CrossRef] [PubMed]
- Sitruk-Ware, R. Hormonal replacement therapy. Rev. Endocr. Metab. Disorder. 2002, 3, 243–256. [Google Scholar] [CrossRef]
- Daniel, J.M.; Fader, A.J.; Spencer, A.L.; Dohanich, G.P. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm. Behav. 1997, 32, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Dohanich, G.P.; Fader, A.J.; Javorsky, D.J. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behav. Neurosci. 1994, 108, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Galea, L.A.; Wide, J.K.; Paine, T.A.; Holmes, M.M.; Ormerod, B.K.; Floresco, S.B. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav. Brain Res. 2001, 126, 115–126. [Google Scholar] [CrossRef]
- Holmes, M.C.; Wide, J.K.; Galea, L.A.M. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav. Neurosci. 2002, 116, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Luine, V.N.; Richards, S.T.; Wu, V.Y.; Beck, K.D. Estradiol enhances learning and memory in a spatial memory task and effects of monoaminergic neurotransmitters. Horm. Behav. 1998, 34, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Luine, V.N.; Jacome, L.F.; Maclusky, N.J. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 2003, 144, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Marriott, L.K.; Korol, D.L. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol. Learn. Memory 2003, 80, 315–322. [Google Scholar] [CrossRef]
- McLaughlin, K.J.; Bimonte-Nelson, H.A.; Neisewander, J.L.; Conrad, C.D. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Horm. Behav. 2008, 54, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Packard, M.G.; Teather, L.A. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol. Learn. Memory 1997, 68, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, N.J.; Williams, C.L. Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci. 2001, 115, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Meyer, E.M.; Millard, W.J.; Simpkins, J.W. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994, 644, 305–312. [Google Scholar] [CrossRef]
- Fernandez, S.M.; Frick, K.M. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav. Neurosci. 2004, 118, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.C.; Kerr, K.M.; Orr, P.T.; Frick, K.M. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav. Neurosci. 2008, 122, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, N.J.; Squire, L.R.; Clark, R.E. Spatial memory, recognition memory, and the hippocampus. Proc. Nat. Acad. Sci. USA 2004, 101, 14515–14520. [Google Scholar] [CrossRef] [PubMed]
- Forwood, S.E.; Winters, B.D.; Bussey, T.J. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus 2005, 15, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.S.; Tull, L.E.; Stackman, R.W. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol. Learn. Memory 2004, 82, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.M.; Lewis, M.C.; Pechenino, A.S.; Harburger, L.L.; Orr, P.T.; Gresack, J.E.; Schafe, G.E.; Frick, K.M. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J. Neurosci. 2008, 28, 8660–8667. [Google Scholar] [CrossRef] [PubMed]
- Voytko, M.L. Estrogen and the cholinergic system modulate visuospatial attention in monkeys (Macaca fascicularis). Behav. Neurosci. 2002, 116, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Voytko, M.L. The effects of long-term ovariectomy and estrogen replacement therapy on learning and memory in monkeys (Macaca fascicularis). Behav. Neurosci. 2000, 114, 1078–1187. [Google Scholar] [CrossRef] [PubMed]
- Lacreuse, A.; Herndon, J.G. Estradiol selectively affects processing of conspecifics’ faces in female rhesus monkeys. Psychoneuroendocrinology 2003, 28, 885–905. [Google Scholar] [CrossRef]
- Blaustein, J.D. An estrogen by any other name. Endocrinology 2008, 149, 2697–2698. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.M. Effects of oestrogen on cognition: What have we learned from basic research? J. Neuroendocr. 2006, 18, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, B.M.; Watson, R.I. An evaluation of psychologic effects of sex hormone administration in aged women. I. Results of therapy after six months. J. Gerontol. 1952, 7, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.L.; Stefanick, M.L.; Stafford, R.S. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. J. Am. Med. Assoc. 2004, 291, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bhavnani, B.R. Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer’s. J. Steroid Biochem. Mol. Biol. 2003, 85, 473–482. [Google Scholar] [CrossRef]
- Campbell, S.; Whitehead, M. Oestrogen therapy and the menopausal syndrome. Clin. Obstet. Gynaecol. 1977, 4, 31–47. [Google Scholar] [PubMed]
- Kantor, H.I.; Michael, C.M.; Shore, H. Estrogen for older women. Am. J. Obstet. Gynecol. 1973, 116, 115–158. [Google Scholar] [CrossRef]
- Shumaker, S.A.; Legault, C.; Rapp, S.R.; Thal, L.; Wallace, R.B.; Ockene, J.K.; Hendrix, S.L.; Jones, B.N., 3rd; Assaf, A.R.; Jackson, R.D.; Kotchen, J.M.; Wassertheil-Smoller, S.; Wactawski-Wende, J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. J. Am. Med. Assoc. 2003, 289, 2651–2662. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Rapp, S.R.; Shumaker, S.A.; Brunner, R.; Manson, J.E.; Sherwin, B.B.; Hsia, J.; Margolis, K.L.; Hogan, P.E.; Wallace, R.; Dailey, M.; Freeman, R.; Hays, J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. J. Am. Med. Assoc. 2004, 291, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, S.A.; Legault, C.; Kuller, L.; Rapp, S.R.; Thal, L.; Lane, D.S.; Fillit, H.; Stefanick, M.L.; Hendrix, S.L.; Lewis, C.E.; Masaki, K.; Coker, L.H. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. J. Am. Med. Assoc. 2004, 291, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Resnick, S.M.; Maki, P.M.; Rapp, S.R.; Espeland, M.A.; Brunner, R.; Coker, L.H.; Granek, I.A.; Hogan, P.; Ockene, J.K.; Shumaker, S.A. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J. Clin. Endocrinol. Metab. 2006, 91, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Resnick, S.M.; Espeland, M.A.; Jaramillo, S.A.; Hirsch, C.; Stefanick, M.L.; Murray, A.M.; Ockene, J.; Davatzikos, C. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology 2009, 72, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, S.A.; Reboussin, B.A.; Espeland, M.A.; Rapp, S.R.; McBee, W.L.; Dailey, M.; Bowen, D.; Terrell, T.; Jones, B.N. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin. Trials 1998, 19, 604–621. [Google Scholar] [CrossRef]
- Bohacek, J.; Bearl, A.M.; Daniel, J.M. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J. Neuroendocrinol. 2008, 20, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Woolley, C.S. Effects of oestradiol on hippocampal circuitry. Novart. Fdn. Symp. 2000, 230, 173–180, discussion 181-187. [Google Scholar]
- Voytko, M.L.; Murray, R.; Higgs, C.J. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J. Neurosci. 2009, 29, 10362–10370. [Google Scholar] [CrossRef] [PubMed]
- Voytko, M.L.; Higgs, C.J.; Murray, R. Differential effects on visual and spatial recognition memory of a novel hormone therapy regimen of estrogen alone or combined with progesterone in older surgically menopausal monkeys. Neuroscience 2008, 154, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Rapp, P.R.; Morrison, J.H.; Roberts, J.A. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J. Neurosci. 2003, 23, 5708–5714. [Google Scholar] [CrossRef] [PubMed]
- Lacreuse, A.; Wilson, M.E.; Herndon, J.G. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol. Aging 2002, 23, 589–600. [Google Scholar] [CrossRef]
- Luine, V.; Rodriguez, M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav. Neural Biol. 1994, 62, 230–236. [Google Scholar] [CrossRef]
- Ziegler, D.R.; Gallagher, M. Spatial memory in middle-aged female rats: Assessment of estrogen replacement after ovariectomy. Brain Res. 2005, 1052, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Beason-Held, L.L.; Rosene, D.L.; Killiany, R.J.; Moss, M.B. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus 1999, 9, 562–574. [Google Scholar] [CrossRef]
- Moss, M.B.; Killiany, R.J.; Lai, Z.C.; Rosene, D.L.; Herndon, J.G. Recognition memory span in rhesus monkeys of advanced age. Neurobiol. Aging 1997, 18, 13–19. [Google Scholar] [CrossRef]
- Acosta, J.I.; Mayer, L.; Talboom, J.S.; Zay, C.; Scheldrup, M.; Castillo, J.; Demers, L.M.; Enders, C.K.; Bimonte-Nelson, H.A. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm. Behav. 2009, 55, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Bohacek, J.; Daniel, J.M. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm. Behav. 2007, 52, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.M.; Fernandez, S.M.; Bennett, J.C.; Prange-Kiel, J.; MacLusky, N.J.; Leranth, C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur. J. Neurosci. 2004, 19, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Bagger, Y.Z.; Tanko, L.B.; Alexandersen, P.; Qin, G.; Christiansen, C. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause 2005, 12, 12–17. [Google Scholar] [CrossRef] [PubMed]
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause 2010, 17, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Stovall, D.W.; Beard, M.K.; Barbier, S.; Chen, E.; Rosenberg, E.; de Papp, A.E. Response to oral bisphosphonates in subgroups of younger and older postmenopausal women. J. Womens Health (Larchmt) 2010, 19, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr. Rev. 2010, 31, 224–53. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Prentice, R.; Thompson, D.J.; Herrmann, W.L. Association of exogenous estrogen and endometrial carcinoma. N. Engl. J. Med. 1975, 293, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Chesler, E.J.; Juraska, J.M. Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Horm. Behav. 2000, 38, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Brett, M.; Baxendale, S. Motherhood and memory: A review. Psychoneuroendocrinology 2001, 26, 339–362. [Google Scholar] [CrossRef]
- Freeman, E.W.; Weinstock, L.; Rickels, K.; Sondheimer, S.J.; Coutifaris, C. A placebo-controlled study of effects of oral progesterone on performance and mood. Br. J. Clin. Pharmacol. 1992, 33, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Hopper, B.R.; Vargo, T.M.; Yen, S.S. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol. Reprod. 1979, 21, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Steger, R.W.; Bruni, J.F.; Meites, J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology 1978, 103, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M.; Ratner, A. Effect of ovariectomy on plasma LH, FSH, estradiol, and progesterone and medial basal hypothalamic LHRH concentrations old and young rats. Neuroendocrinology 1980, 30, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.G.; Juraska, J.M. Sex differences and estropausal phase effects on water maze performance in aged rats. Neurobiol. Learn. Memory 2000, 74, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.G.; Juraska, J.M. Spatial and nonspatial learning across the rat estrous cycle. Behav. Neurosci. 1997, 111, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Berry, B.; McMahan, R.; Gallagher, M. Spatial learning and memory at defined points of the estrous cycle: Effects on performance of a hippocampal-dependent task. Behav. Neurosci. 1997, 111, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Stackman, R.W.; Blasberg, M.E.; Langan, C.J.; Clark, A.S. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol. Learn. Memory 1997, 67, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Harburger, L.L.; Bennett, J.C.; Frick, K.M. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol. Aging 2007, 28, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Bimonte-Nelson, H.A.; Nelson, M.E.; Granholm, A.C. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport 2004, 15, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.C.; Baudry, M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur. J. Neurosci. 2009, 29, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, J.; Brinton, R.D. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology 2002, 143, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.; Fahim, G. Do Depot Medroxyprogesterone Acetate Contraceptive Injections Cause Mood Changes and Memory Impairment? Prim. Psychiatr. 2005, 12, 59–60. [Google Scholar]
- Ziel, H.K.; Finkle, W.D. Increased risk of endometrial carcinoma among users of conjugated estrogens. N. Engl. J. Med. 1975, 293, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Prokai, L.; Prokai-Tatrai, K.; Perjesi, P.; Zharikova, A.D.; Perez, E.J.; Liu, R.; Simpkins, J.W. Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc. Nat. Acad. Sci. USA 2003, 100, 11741–11746. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.S. Memory loss as a reported symptom of andropause. Arch. Androl. 2001, 47, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.M.; Chen, J.J.; Crapo, L.; Gray, G.D.; Greenleaf, W.J.; Catania, J.A. Hormonal changes and sexual function in aging men. J. Clin. Endocrinol. Metab. 1983, 57, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, R.A.; Cunningham, G.R. Andropause: is androgen replacement therapy indicated for the aging male? Annu. Rev. Med. 2005, 56, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, E.; Williams, J.; Budge, M.; Barnetson, L.; Combrinck, M.; Smith, A.D. Serum total testosterone is lower in men with Alzheimer’s disease. Neurol. Endocrinol. Lett. 2001, 22, 163–168. [Google Scholar]
- Moffat, S.D.; Zonderman, A.B.; Metter, E.J.; Blackman, M.R.; Harman, S.M.; Resnick, S.M. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J. Clin. Endocrinol. Metab. 2002, 87, 5001–5007. [Google Scholar] [CrossRef] [PubMed]
- Moffat, S.D.; Zonderman, A.B.; Metter, E.J.; Kawas, C.; Blackman, M.R.; Harman, S.M.; Resnick, S.M. Free testosterone and risk for Alzheimer disease in older men. Neurology 2004, 62, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Rosario, E.R.; Chang, L.; Stanczyk, F.Z.; Pike, C.J. Age-related testosterone depletion and the development of Alzheimer disease. J. Am. Med. Assoc. 2004, 292, 1431–1432. [Google Scholar] [CrossRef] [PubMed]
- Kimura, D.; Hampson, E. Cognitive pattern in men and women is influenced by fluctuations in sex hormones. Curr. Dir. Psychol. Sci. 1994, 3, 57–61. [Google Scholar] [CrossRef]
- Neave, N.; Menaged, M.; Weightman, D.R. Sex differences in cognition: the role of testosterone and sexual orientation. Brain Cogn. 1999, 41, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Silverman, I.; Kastuk, D.; Choi, J.; Phillips, K. Testosterone levels and spatial ability in men. Psychoneuroendocrinology 1999, 24, 813–822. [Google Scholar] [CrossRef]
- Barrett-Connor, E.; Goodman-Gruen, D.; Patay, B. Endogenous sex hormones and cognitive function in older men. J. Clin. Endocr. Metab. 1999, 84, 3681–3685. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Lui, L.Y.; Zmuda, J.; Cauley, J. Sex hormones and cognitive function in older men. J. Am. Geriatr. Soc. 2002, 50, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Gouchie, C.; Kimura, D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology 1991, 16, 323–334. [Google Scholar] [CrossRef]
- Cherrier, M.M.; Matsumoto, A.M.; Amory, J.K.; Johnson, M.; Craft, S.; Peskind, E.R.; Raskind, M.A. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology 2007, 32, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Wittert, G.; Burns, N.R.; McPherson, J. Endogenous testosterone levels, mental rotation performance, and constituent abilities in middle-to-older aged men. Horm. Behav. 2008, 53, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Lacreuse, A.; Chiavetta, M.R.; Shirai, A.A.; Meyer, J.S.; Grow, D.R. Effects of testosterone on cognition in young adult male rhesus monkeys. Physiol. Behav. 2009, 98, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M.M.; Asthana, S.; Plymate, S.; Baker, L.; Matsumoto, A.M.; Peskind, E.; Raskind, M.A.; Brodkin, K.; Bremner, W.; Petrova, A.; LaTendresse, S.; Craft, S. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 2001, 57, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.B.; Singh, A.B.; Woodhouse, L.J.; Storer, T.W.; Casaburi, R.; Dzekov, J.; Dzekov, C.; Sinha-Hikim, I.; Bhasin, S. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. J. Clin. Endocrinol. Metab. 2005, 90, 3838–3846. [Google Scholar] [CrossRef] [PubMed]
- Janowsky, J.S.; Oviatt, S.K.; Orwoll, E.S. Testosterone influences spatial cognition in older men. Behav. Neurosci. 1994, 108, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Woodhouse, L.; Casaburi, R.; Singh, A.B.; Bhasin, D.; Berman, N.; Chen, X.; Yarasheski, K.E.; Magliano, L.; Dzekov, C.; Dzekov, J.; Bross, R.; Phillips, J.; Sinha-Hikim, I.; Shen, R.; Storer, T.W. Testosterone dose-response relationships in healthy young men. Amer. J. Physiol., Endocrinol. Metab. 2001, 281, E1172–E1181. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M.M.; Matsumoto, A.M.; Amory, J.K.; Ahmed, S.; Bremner, W.; Peskind, E.R.; Raskind, M.A.; Johnson, M.; Craft, S. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology 2005, 64, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.S.; Pu, S.J. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male 2003, 6, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.M.; Fabregas, G.; Song, C.; Biskup, B.; Bellantonio, S. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J. Gerontol. Biol. Sci. Med. Sci. 2004, 59, 75–78. [Google Scholar] [CrossRef]
- Becker, K. Principles and Practice of Endocrinology and Metabolism; J.B. Lippincott Co.: Philadelphia, PA, USA, 1995. [Google Scholar]
- Naftolin, F. Brain aromatization of androgens. J. Reprod. Med. 1994, 39, 257–261. [Google Scholar] [PubMed]
- Bimonte-Nelson, H.A.; Singleton, R.S.; Nelson, M.E.; Eckman, C.B.; Barber, J.; Scott, T.Y.; Granholm, A.C. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp. Neurol. 2003, 181, 301–312. [Google Scholar] [CrossRef]
- Benice, T.S.; Raber, J. Testosterone and dihydrotestosterone differentially improve cognition in aged female mice. Learn. Memory 2009, 16, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, H.; Hiatt, E.S.; Lei, Z.M.; Rao, C.V. Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm. Behav. 1995, 29, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Gao, G.; Huang, W. A study on co-localization of FSH and its receptor in rat hippocampus. J. Mol. Histol. 2008, 39, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Webber, K.M.; Casadesus, G.; Bowen, R.L.; Perry, G.; Smith, M.A. Evidence for the role of luteinizing hormone in Alzheimer disease. Endocr. Metab. Immun. Disord. Drug Target. 2007, 7, 300–303. [Google Scholar] [CrossRef]
- Hyde, Z.; Flicker, L.; Almeida, O.P.; McCaul, K.A.; Jamrozik, K.; Hankey, G.J.; Chubb, S.A.; Yeap, B.B. Higher luteinizing hormone is associated with poor memory recall: the health in men study. J. Alzheimers Dis. 2010, 19, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Webber, K.M.; Perry, G.; Smith, M.A.; Casadesus, G. The contribution of luteinizing hormone to Alzheimer disease pathogenesis. Clin. Med. Res. 2007, 5, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.M.; Rao, C.V.; Kornyei, J.L.; Licht, P.; Hiatt, E.S. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology 1993, 132, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lei, Z.M.; Rao, C.V. Immortalized hippocampal cells contain functional luteinizing hormone/human chorionic gonadotropin receptors. Life Sci. 1999, 65, 2083–2098. [Google Scholar] [CrossRef]
- Martinez-Morales, J.R.; Lopez-Coviella, I.; Hernandez-Jimenez, J.G.; Reyes, R.; Bello, A.R.; Hernandez, G.; Blusztajn, J.K.; Alonso, R. Sex steroids modulate luteinizing hormone-releasing hormone secretion in a cholinergic cell line from the basal forebrain. Neuroscience 2001, 103, 1025–1031. [Google Scholar] [CrossRef]
- Nauton, P.; Giry, N.; Bruhat, M.A.; Alliot, J. Effect of administration of an analog of LHRH on appetitive learning in young and middle-aged female rats. Pharmacol. Biochem. Behav. 1992, 43, 1005–1013. [Google Scholar] [CrossRef]
- Alliot, J.; Nauton, P.; Bruhat, M.A. Administration of LHRH analog can improve working memory in aged female rats. Psychoneuroendocrinology 1993, 18, 543–550. [Google Scholar] [CrossRef]
- Bowen, R.L.; Verdile, G.; Liu, T.; Parlow, A.F.; Perry, G.; Smith, M.A.; Martins, R.N.; Atwood, C.S. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J. Biol. Chem. 2004, 279, 20539–20545. [Google Scholar] [CrossRef] [PubMed]
- Casadesus, G.; Webber, K.M.; Atwood, C.S.; Pappolla, M.A.; Perry, G.; Bowen, R.L.; Smith, M.A. Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochem. Biophys. Acta 2006, 1762, 447–452. [Google Scholar]
- Casadesus, G.; Milliken, E.L.; Webber, K.M.; Bowen, R.L.; Lei, Z.; Rao, C.V.; Perry, G.; Keri, R.A.; Smith, M.A. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol. Cell. Biochem. 2007, 269, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Short, R.A.; Bowen, R.L.; O’Brien, P.C.; Graff-Radford, N.R. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin. Proc. 2001, 76, 906–909. [Google Scholar] [CrossRef]
- McEwen, B.S.; Coirini, H.; Westlind-Danielsson, A.; Frankfurt, M.; Gould, E.; Schumacher, M.; Woolley, C. Steroid hormones as mediators of neural plasticity. J. Steroid Biochem. Mol. Biol. 1991, 39, 223–232. [Google Scholar] [CrossRef]
- McEwen, B.S. Multiple ovarian hormone effects on brain structure and function. J. Gend. Specif. Med. 1998, 1, 33–41. [Google Scholar] [PubMed]
- Moffat, S.D. Effects of testosterone on cognitive and brain aging in elderly men. Ann. N Y Acad. Sci. 2005, 1055, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.R.; Pluchino, N.; Luisi, S.; Luisi, M. Estrogen, cognition and female ageing. Hum. Reprod. Update 2007, 13, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Brody, H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J. Comp. Neurol. 1955, 102, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Simic, G.; Kostovic, I.; Winblad, B.; Bogdanovic, N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J. Comp. Neurol. 1997, 379, 482–494. [Google Scholar] [CrossRef]
- West, M.J. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol. Aging 1993, 14, 287–293. [Google Scholar] [CrossRef]
- Smith, D.E.; Rapp, P.R.; McKay, H.M.; Roberts, J.A.; Tuszynski, M.H. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J. Neurosci. 2004, 24, 4373–4381. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, S.; Bruce, C.J.; Goldman-Rakic, P.S. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic "scotomas". J. Neurosci. 1993, 13, 1479–1497. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.; Fuster, J.M. From perception to action: temporal integrative functions of prefrontal and parietal neurons. Cereb. Cortex 1999, 9, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Goldman-Rakic, P.S. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp. Brain Res. 2000, 133, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.B.; Toni, I.; Josephs, O.; Frackowiak, R.S.; Passingham, R.E. The prefrontal cortex: response selection or maintenance within working memory? Science 2000, 288, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. Frontal lobe and cognitive development. J. Neurocytol. 2002, 31, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Rapp, P.R.; Deroche, P.S.; Mao, Y.; Burwell, R.D. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cereb. Cortex 2002, 12, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.S.; Hinds, J.W. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science 1977, 197, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Kornack, D.R.; Rakic, P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Nat. Acad. Sci. USA 1999, 96, 5768–5773. [Google Scholar] [CrossRef] [PubMed]
- Bertoni-Freddari, C.; Fattoretti, P.; Solazzi, M.; Giorgetti, B.; Di Stefano, G.; Casoli, T.; Meier-Ruge, W. Neuronal death versus synaptic pathology in Alzheimer’s disease. Ann. NY Acad. Sci. 2003, 1010, 635–638. [Google Scholar] [CrossRef]
- Esiri, M. The neuropathology of Alzheimer’s disease. In Neurobiology of Alzheimer’s Disease, 2nd ed.; Dawbarn, D., Allen, S., Eds.; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Poucet, B. A further characterization of the spatial problem-solving deficit induced by lesions of the medial frontal cortex in the rat. Behav. Brain Res. 1990, 41, 229–237. [Google Scholar] [CrossRef]
- Poucet, B.; Herrmann, T. Septum and medial frontal cortex contribution to spatial problem-solving. Behav. Brain Res. 1990, 37, 269–280. [Google Scholar] [CrossRef]
- Miotto, E.C.; Bullock, P.; Polkey, C.E.; Morris, R.G. Spatial working memory and strategy formation in patients with frontal lobe excisions. Cortex 1996, 32, 613–630. [Google Scholar] [CrossRef]
- Ranganath, C.; Johnson, M.K.; D’Esposito, M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia 2003, 41, 378–389. [Google Scholar] [CrossRef]
- Woolley, C.S. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 657–680. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Neural gonadal steroid actions. Science 1981, 211, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.; McEwen, B.S.; Pfaff, D.W. A discontinuous schedule of estradiol treatment is sufficient to activate progesterone-facilitated feminine sexual behavior and to increase cytosol receptors for progestins in the hypothalamus of the rat. Endocrinology 1982, 110, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, D.W.; McEwen, B.S. Actions of estrogens and progestins on nerve cells. Science 1983, 219, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, E.; Timiras, P.S. Electrical activity during the estrous cycle of the rat: cyclic changes in limbic structures. Endocrinology 1968, 83, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Foy, M.R. 17β-estradiol: effect on CA1 hippocampal synaptic plasticity. Neurobiol. Learn. Memory 2001, 76, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Woolley, C.S.; Frankfurt, M.; McEwen, B.S. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 1990, 10, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Mello, L.E.; Freymuller, E.; Haidar, M.A.; Baracat, E.C. Estrogen, progestogen and tamoxifen increase synaptic density of the hippocampus of ovariectomized rats. Neurosci. Lett. 2000, 291, 183–186. [Google Scholar] [CrossRef]
- Woolley, C.S.; McEwen, B.S. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 1992, 12, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Woolley, C.S.; McEwen, B.S. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 1993, 336, 293–306. [Google Scholar] [CrossRef] [PubMed]
- MacLusky, N.J.; Hajszan, T.; Prange-Kiel, J.; Leranth, C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience 2006, 138, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.B. Making more synapses: a way to store information? Cell. Mol. Life Sci. 1999, 55, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Geinisman, Y. Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cereb. Cortex 2000, 10, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Sorra, K.E.; Harris, K.M. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus 2000, 10, 501–511. [Google Scholar] [CrossRef]
- Nimchinsky, E.A.; Sabatini, B.L.; Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002, 64, 313–353. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Matsuzaki, M.; Noguchi, J.; Yasumatsu, N.; Nakahara, H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003, 26, 360–368. [Google Scholar] [CrossRef]
- Sandstrom, N.J.; Williams, C.L. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm. Behav. 2004, 45, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.W. A comparative review of rodent prefrontal cortex and working memory. Curr. Mol. Med. 2002, 2, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.J.; Johnson, P.E.; Dohanich, G.P. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze. Pharmacol. Biochem. Behav. 1999, 62, 711–717. [Google Scholar] [CrossRef]
- Hruska, Z.; Dohanich, G.P. The effects of chronic estradiol treatment on working memory deficits induced by combined infusion of beta-amyloid (1-42) and ibotenic acid. Horm. Behav 2007, 52, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Luine, V.N. Steroid hormone influences on spatial memory. Ann. NY Acad. Sci. 1994, 743, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Lacreuse, A.; Verreault, M.; Herndon, J.G. Fluctuations in spatial recognition memory across the menstrual cycle in female rhesus monkeys. Psychoneuroendocrinology 2001, 26, 623–639. [Google Scholar] [CrossRef]
- Hao, J.; Janssen, W.G.; Tang, Y.; Roberts, J.A.; McKay, H.; Lasley, B.; Allen, P.B.; Greengard, P.; Rapp, P.R.; Kordower, J.H.; Hof, P.R.; Morrison, J.H. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J. Comp. Neurol. 2003, 465, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Janssen, W.G.; Hao, J.; Roberts, J.A.; McKay, H.; Lasley, B.; Allen, P.B.; Greengard, P.; Rapp, P.R.; Kordower, J.H.; Hof, P.R.; Morrison, J.H. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb. Cortex 2004, 14, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Rapp, P.R.; Leffler, A.E.; Leffler, S.R.; Janssen, W.G.; Lou, W.; McKay, H.; Roberts, J.A.; Wearne, S.L.; Hof, P.R.; Morrison, J.H. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J. Neurosci. 2006, 26, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm. Behav. 2002, 42, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm. Behav. 2007, 52, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.A.; McMillan, P.J.; Dobie, D.J.; Dorsa, D.M. Effects of estrogen replacement on choline acetyltransferase and trkA mRNA expression in the basal forebrain of aged rats. Brain Res. 1998, 789, 343–346. [Google Scholar] [CrossRef]
- Kompoliti, K.; Chu, Y.; Polish, A.; Roberts, J.; McKay, H.; Mufson, E.J.; Leurgans, S.; Morrison, J.H.; Kordower, J.H. Effects of estrogen replacement therapy on cholinergic basal forebrain neurons and cortical cholinergic innervation in young and aged ovariectomized rhesus monkeys. J. Comp. Neurol. 2004, 472, 193–207. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, C.A.; Hautamaki, R.D.; Kelley, M.; Meyer, E.M. Effects of ovariectomy and estradiol benzoate on high affinity choline uptake, ACh synthesis, and release from rat cerebral cortical synaptosomes. Brain Res. 1987, 403, 389–92. [Google Scholar] [CrossRef]
- Luine, V.N.; McEwen, B.S. Sex differences in cholinergic enzymes of diagonal band nuclei in the rat preoptic area. Neuroendocrinology 1983, 36, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Luine, V.N. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp. Neurol. 1985, 89, 484–490. [Google Scholar] [CrossRef]
- McMillan, P.J.; Singer, C.A.; Dorsa, D.M. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female Sprague-Dawley rat. J. Neurosci. 1996, 16, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B.; Wu, D.; Hersh, L.B.; Pfaff, D.W. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp. Neurol. 1994, 129, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B.; Pfaff, D.W. Effects of estrogen and fimbria/fornix transection on p75NGFR and ChAT expression in the medial septum and diagonal band of Broca. Exp. Neurol. 1992, 116, 23–39. [Google Scholar] [CrossRef]
- Gibbs, R.B. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Res. 1997, 757, 10–16. [Google Scholar] [CrossRef]
- Granholm, A.C.; Ford, K.A.; Hyde, L.A.; Bimonte, H.A.; Hunter, C.L.; Nelson, M.; Albeck, D.; Sanders, L.A.; Mufson, E.J.; Crnic, L.S. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome. Physiol. Behav. 2002, 77, 371–385. [Google Scholar] [CrossRef]
- Gibbs, R.B. Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: effects of estrogen and progesterone. J. Neurosci. 1996, 16, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B.; Nelson, D.; Anthony, M.S.; Clarkson, T.B. Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomologus monkeys. Neuroscience 2002, 113, 907–914. [Google Scholar] [CrossRef]
- Browne, C.; Tobin, J.R.; Voytko, M.L. Effects of two years of conjugated equine estrogens on cholinergic neurons in young and middle-aged ovariectomized monkeys. Brain Res. 2009, 1264, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Tinkler, G.P.; Tobin, J.R.; Voytko, M.L. Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J. Comp. Neurol. 2004, 469, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, J.D. Estrogen receptor immunoreactivity in rat brain: rapid effects of estradiol injection. Endocrinology 1993, 132, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Kassis, J.A.; Gorski, J. Estrogen receptor replenishment. Evidence for receptor recycling. J. Biol. Chem. 1981, 256, 7378–7382. [Google Scholar] [PubMed]
- Rosser, M.; Chorich, L.; Howard, E.; Zamorano, P.; Mahesh, V.B. Changes in rat uterine estrogen receptor messenger ribonucleic acid levels during estrogen- and progesterone-induced estrogen receptor depletion and subsequent replenishment. Biol. Reprod. 1993, 48, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience 2000, 101, 931–938. [Google Scholar] [CrossRef]
- Nakamura, N.; Fujita, H.; Kawata, M. Effects of gonadectomy on immunoreactivity for choline acetyltransferase in the cortex, hippocampus, and basal forebrain of adult male rats. Neuroscience 2002, 109, 473–485. [Google Scholar] [CrossRef]
- Romeo, R.D.; Staub, D.; Jasnow, A.M.; Karatsoreos, I. N.; Thornton, J.E.; McEwen, B.S. Dihydrotestosterone increases hippocampal N-methyl-D-aspartate binding but does not affect choline acetyltransferase cell number in the forebrain or choline transporter levels in the CA1 region of adult male rats. Endocrinology 2005, 146, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.R.; Gore, A.C. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp. Biol. Med. (Maywood) 2004, 229, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Beato, M.; Chavez, S.; Truss, M. Transcriptional regulation by steroid hormones. Steroids 1996, 61, 240–251. [Google Scholar] [CrossRef]
- Toran-Allerand, C.D. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann. NY Acad. Sci. 2005, 1052, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Arterburn, J.B.; Smith, H.O.; Oprea, T.I.; Sklar, L.A.; Hathaway, H.J. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu. Rev. Physiol. 2008, 70, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.; Mauk, R.; Ninaci, D.; Nelson, D.; Gibbs, R.B. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm. Behav. 2009, 56, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Prokai, L.; Simpkins, J.W. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol. Ther. 2007, 114, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, N.; Pfaff, D.W. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008, 29, 238–257. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D.; Tran, J.; Proffitt, P.; Montoya, M. 17 beta-Estradiol enhances the outgrowth and survival of neocortical neurons in culture. Neurochem. Res. 1997, 22, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Diaz Brinton, R.; Chen, S.; Montoya, M.; Hsieh, D.; Minaya, J.; Kim, J.; Chu, H.P. The women’s health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiol. Aging 2000, 21, 475–496. [Google Scholar] [CrossRef]
- Brinton, R.D.; Chen, S.; Montoya, M.; Hsieh, D.; Minaya, J. The estrogen replacement therapy of the Women’s Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer’s disease. Maturitas 2000, 34, S35–S52. [Google Scholar] [CrossRef]
- Littleton-Kearney, M.T.; Klaus, J.A.; Hurn, P.D. Effects of combined oral conjugated estrogens and medroxyprogesterone acetate on brain infarction size after experimental stroke in rat. J. Cereb. Blood Flow Metab. 2005, 25, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Brinton, R.D. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer’s disease. BMC Neurosci. 2006, 7, 24. [Google Scholar]
- Zemlyak, I.; Brooke, S.M.; Sapolsky, R.M. Protection against gp120-induced neurotoxicity by an array of estrogenic steroids. Brain Res. 2002, 958, 272–276. [Google Scholar] [CrossRef]
- Brinton, R.D.; Proffitt, P.; Tran, J.; Luu, R. Equilin, a principal component of the estrogen replacement therapy premarin, increases the growth of cortical neurons via an NMDA receptor-dependent mechanism. Exp. Neurol. 1997, 147, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, J.; Morales, A.; Brinton, R.D. Medroxyprogesterone acetate exacerbates glutamate excitotoxicity. Gynecol. Endocrinol. 2006, 22, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Le, Q.; Goodyer, C.; Gelfand, M.; Trifiro, M.; LeBlanc, A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem. 2001, 77, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Champagne, N.; Beitel, L.K.; Goodyer, C.G.; Trifiro, M.; LeBlanc, A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. J. Neurosci. 2004, 24, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Yao, M.; Pike, C.J. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J. Neurochem. 2005, 94, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Chisu, V.; Manca, P.; Lepore, G.; Gadau, S.; Zedda, M.; Farina, V. Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3-nitro-L-tyrosine incorporation into alpha-tubulin in a mouse neuroblastoma cell line. Arch. Ital. Biol. 2006, 144, 63–73. [Google Scholar]
- Azcoitia, I.; Sierra, A.; Veiga, S.; Honda, S.; Harada, N.; Garcia-Segura, L.M. Brain aromatase is neuroprotective. J. Neurobiol. 2001, 47, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Veiga, S.; Garcia-Segura, L.M.; Azcoitia, I. Neuroprotection by the steroids pregnenolone and dehydroepiandrosterone is mediated by the enzyme aromatase. J. Neurobiol. 2003, 56, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Bimonte-Nelson, H.A.; Granholm, A.C.; Nelson, M.E.; Moore, A.B. Patterns of neurotrophin protein levels in male and female Fischer 344 rats from adulthood to senescence: how young is "young" and how old is "old"? Exp. Aging Res. 2008, 34, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Thomas, K.L.; Everitt, B.J. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat. Neurosci. 2000, 3, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.C. Oestrogen and nerve growth factor - neuroprotection and repair in Alzheimer’s disease. Expert Opin. Investig. Drugs 2000, 9, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Kesslak, J.P.; So, V.; Choi, J.; Cotman, C.W.; Gomez-Pinilla, F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav. Neurosci. 1998, 112, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.J.; Chauhan, N.B. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res. Brain Res. Rev. 2000, 33, 199–227. [Google Scholar] [CrossRef]
- Levi-Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Woolf, N.J. Cholinergic systems in mammalian brain and spinal cord. Prog. Neurobiol. 1991, 37, 475–524. [Google Scholar] [CrossRef]
- Gibbs, R.B. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998, 810, 294. [Google Scholar] [CrossRef]
- Pan, Y.; Anthony, M.; Clarkson, T.B. Effect of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Proc. Soc. Exp. Biol. Med. 1999, 221, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Hafting, T.; Fyhn, M.; Molden, S.; Moser, M.B.; Moser, E.I. Microstructure of a spatial map in the entorhinal cortex. Nature 2005, 436, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Katoh-Semba, R.; Semba, R.; Kato, H.; Ueno, M.; Arakawa, Y.; Kato, K. Regulation by androgen of levels of the beta subunit of nerve growth factor and its mRNA in selected regions of the mouse brain. J. Neurochem. 1994, 62, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Tirassa, P.; Thiblin, I.; Agren, G.; Vigneti, E.; Aloe, L.; Stenfors, C. High-dose anabolic androgenic steroids modulate concentrations of nerve growth factor and expression of its low affinity receptor (p75-NGFr) in male rat brain. J. Neurosci. Res. 1997, 47, 198–207. [Google Scholar] [CrossRef]
- Ottem, E.N.; Beck, L.A.; Jordan, C.L.; Breedlove, S.M. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology 2007, 148, 3655–3665. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Chalifoux, S.; Giesser, B.S.; Voskuhl, R.R. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J. Neuroinflamm. 2008, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.K.; Shen, L.; Ke, H.; Li, F.; Ni, L.M.; Li, Q.H. Effects of androgen on the expression of brain aromatase cytopigment and nerve growth factor in neonatal rats with hypoxic-ischemic brain damage. Zhongguo Dang Dai Er Ke Za Zhi 2008, 10, 441–446. [Google Scholar] [PubMed]
- Eckerman, D.A.; Gordon, W.A.; Edwards, J.D.; MacPhail, R.C.; Gage, M.I. Effects of scopolamine, pentobarbital, and amphetamine on radial arm maze performance in the rat. Pharmacol. Biochem. Behav. 1980, 12, 595–602. [Google Scholar] [CrossRef]
- Rupniak, N.M.; Field, M.J.; Samson, N.A.; Steventon, M.J.; Iversen, S.D. Direct comparison of cognitive facilitation by physostigmine and tetrahydroaminoacridine in two primate models. Neurobiol. Aging 1990, 11, 609–613. [Google Scholar] [CrossRef]
- Drachman, D.A.; Leavitt, J. Human memory and the cholinergic system. A relationship to aging? Arch. Neurol. 1974, 30, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Conner, J.M.; Kordower, J.H. Nerve growth factor in Alzheimer’s disease: defective retrograde transport to nucleus basalis. Neuroreport 1995, 6, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Bimonte-Nelson, H.A.; Hunter, C.L.; Nelson, M.E.; Granholm, A.C. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav. Brain Res. 2003, 139, 47–57. [Google Scholar] [CrossRef]
- Almli, C.R.; Levy, T.J.; Han, B. H.; Shah, A.R.; Gidday, J.M.; Holtzman, D.M. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp. Neurol. 2000, 166, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.E.; Flinn, P.; Bao, J.; Venya, R.; Hayes, R.L. Nerve growth factor attenuates cholinergic deficits following traumatic brain injury in rats. Exp. Neurol. 1997, 146, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Backman, C.; Rose, G.M.; Hoffer, B.J.; Henry, M.A.; Bartus, R.T.; Friden, P.; Granholm, A.C. Systemic administration of a nerve growth factor conjugate reverses age-related cognitive dysfunction and prevents cholinergic neuron atrophy. J. Neurosci. 1996, 16, 5437–5442. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Wictorin, K.; Bjorklund, A.; Williams, L.R.; Varon, S.; Gage, F.H. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 1987, 329, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Gustilo, M.C.; Markowska, A.L.; Breckler, S.J.; Fleischman, C.A.; Price, D.L.; Koliatsos, V.E. Evidence that nerve growth factor influences recent memory through structural changes in septohippocampal cholinergic neurons. J. Comp. Neurol. 1999, 405, 491–507. [Google Scholar] [CrossRef]
- Markowska, A.L.; Koliatsos, V.E.; Breckler, S.J.; Price, D.L.; Olton, D.S. Human nerve growth factor improves spatial memory in aged but not in young rats. J. Neurosci. 1994, 14, 4815–4824. [Google Scholar] [CrossRef] [PubMed]
- Scali, C.; Casamenti, F.; Pazzagli, M.; Bartolini, L.; Pepeu, G. Nerve growth factor increases extracellular acetylcholine levels in the parietal cortex and hippocampus of aged rats and restores object recognition. Neurosci. Lett. 1994, 170, 117–120. [Google Scholar] [CrossRef]
- Smith, Y.R.; Love, T.; Persad, C.C.; Tkaczyk, A.; Nichols, T.E.; Zubieta, J.K. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J. Clin. Endocrinol. Metab. 2006, 91, 4476–4481. [Google Scholar] [CrossRef] [PubMed]






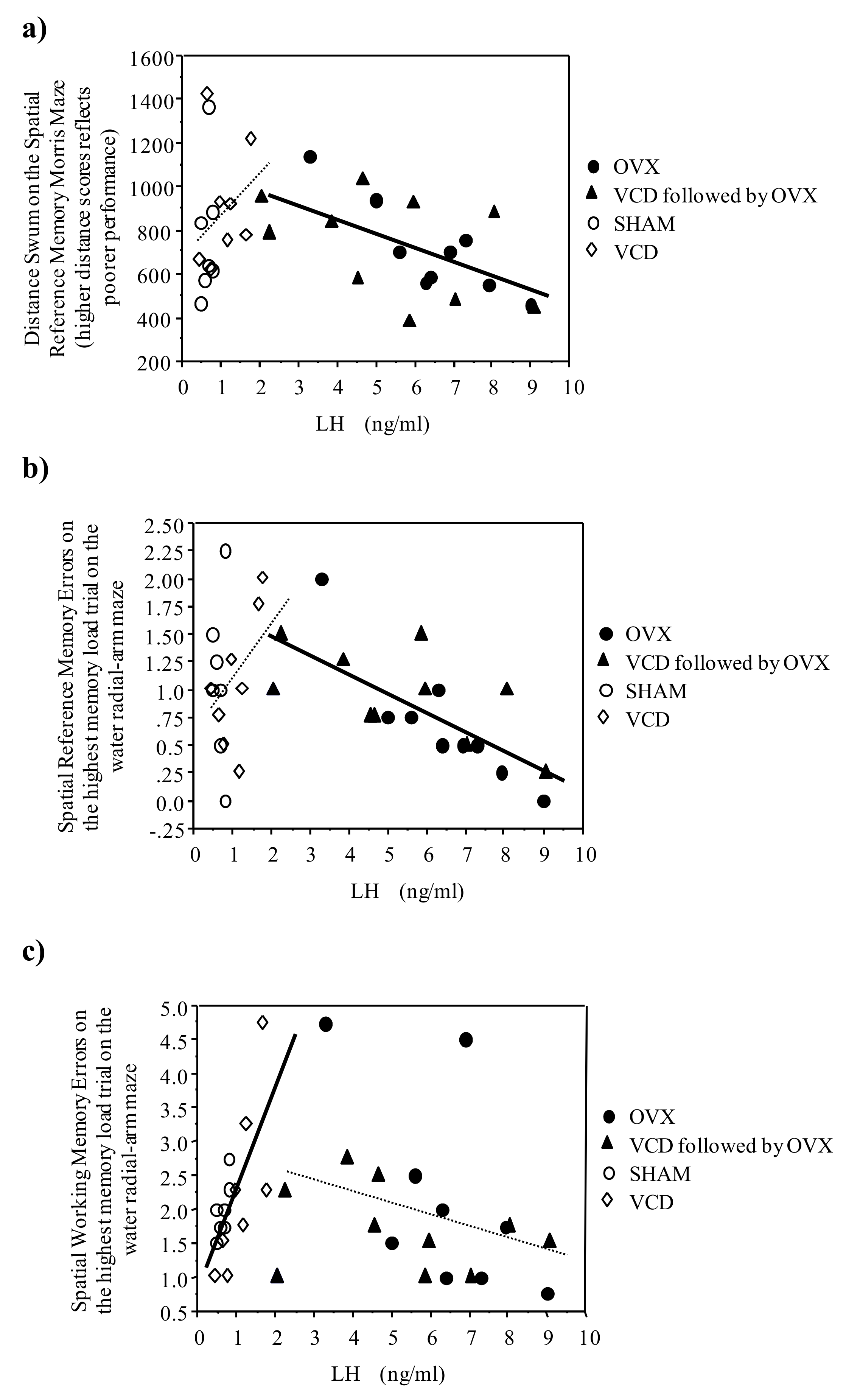
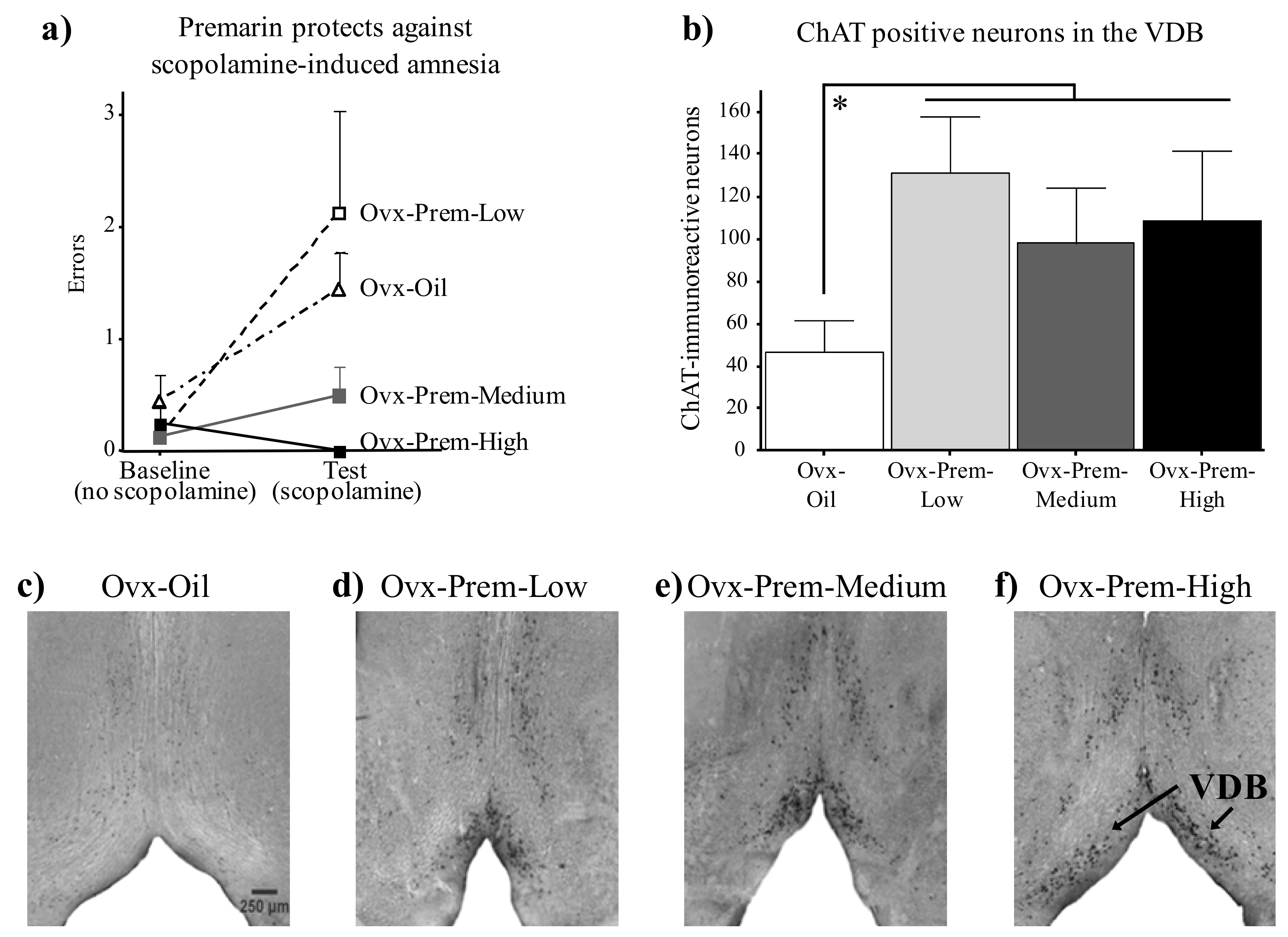
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bimonte-Nelson, H.A.; Acosta, J.I.; Talboom, J.S. Neuroscientists as Cartographers: Mapping the Crossroads of Gonadal Hormones, Memory and Age Using Animal Models. Molecules 2010, 15, 6050-6105. https://doi.org/10.3390/molecules15096050
Bimonte-Nelson HA, Acosta JI, Talboom JS. Neuroscientists as Cartographers: Mapping the Crossroads of Gonadal Hormones, Memory and Age Using Animal Models. Molecules. 2010; 15(9):6050-6105. https://doi.org/10.3390/molecules15096050
Chicago/Turabian StyleBimonte-Nelson, Heather A., Jazmin I. Acosta, and Joshua S. Talboom. 2010. "Neuroscientists as Cartographers: Mapping the Crossroads of Gonadal Hormones, Memory and Age Using Animal Models" Molecules 15, no. 9: 6050-6105. https://doi.org/10.3390/molecules15096050
APA StyleBimonte-Nelson, H. A., Acosta, J. I., & Talboom, J. S. (2010). Neuroscientists as Cartographers: Mapping the Crossroads of Gonadal Hormones, Memory and Age Using Animal Models. Molecules, 15(9), 6050-6105. https://doi.org/10.3390/molecules15096050



