Correlation between Cytotoxic Activities and Reduction Potentials of Heterocyclic Quinones
Abstract
:1. Introduction
2. Results and Discussion

| MG-MID of log GI50 | (μM) | MG-MID of log LC50 | (μM) | |
|---|---|---|---|---|
| 1 | -5.56 | (2.75) | -5.04 | (9.12) |
| 2 | -6.08 | (0.831) | -4.84 | (14.45) |
| 3 | -6.29 | (0.513) | -5.18 | (6.61) |
| 4 | -6.52 | (0.302) | -5.83 | (1.48) |
| 5 | -5.38 | (4.17) | -4.26 | (54.95) |
| 6 | -6.54 | (0.288) | -5.70 | (2.00) |
| 7 | -7.15 | (0.071) | -6.14 | (0.724) |
| 8 | -7.35 | (0.045) | -6.40 | (0.398) |
| 9 | -4.88 | (13.18) | -4.11 | (77.62) |
| 10 | -5.69 | (2.04) | -4.63 | (23.44) |
| 11 | -5.03 | (9.33) | -4.13 | (74.13) |
| 12 | -6.12 | (0.759) | -5.03 | (9.33) |


| Compound | Epc-1 (mV) | Epc-2 (mV) | Epa (mV) |
|---|---|---|---|
| 1 | - 339 | - 426 | - 400 |
| 2 | - 365 | - 435 | - 417 |
| 3 | - 333 | - 429 | - 411 |
| 4 | - 294 | – | - 282 |
| 5 | - 359 | - 245 | - 431 |
| 6 | - 365 | – | - 330 |
| 7 | - 275 | – | - 253 |
| 8 | - 267 | – | - 245 |
| 9 | - 370 | - 245 | - 374 |
| 10 | - 370 | – | - 362 |
| 11 | - 384 | – | – |
| 12 | - 359 | (- 640) | - 336 |
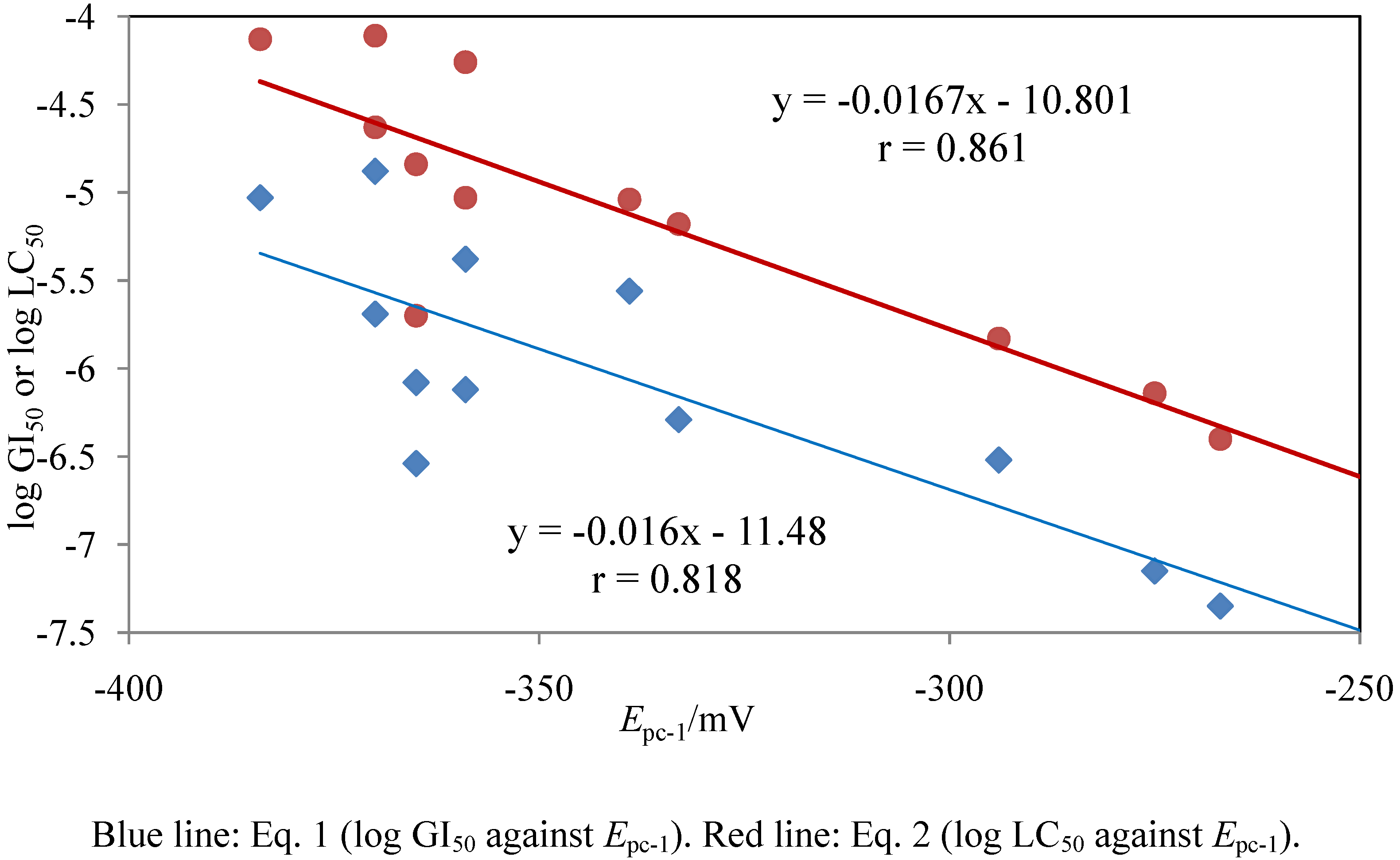
| Steric energy (kcal/mole) | Total energy (eV) | LUMO (eV) | HOMO (eV) | SASA*3 | log P | |
|---|---|---|---|---|---|---|
| 1 | -11.449 | -114.15 | -1.470 | -9.626 | 102.26 | 1.750 |
| 2 | -16.057 | -126.4 | -1.387 | -9.523 | 104.58 | 1.466 |
| 3 | -16.000 | -126.4 | -1.407 | -9.545 | 104.52 | 1.466 |
| 4 | -0.746 | -103.66 | -1.809 | -9.974 | 94.668 | -0.230 |
| 5 | -13.257 | -104.02 | -1.685 | -9.994 | 100.34 | 1.286 |
| 6 | -3.978 | -100.72 | -1.914 | -10.064 | 100.42 | 0.113 |
| 7 | -10.420 | -106.18 | -1.913 | -10.148 | 99.581 | -0.026 |
| 8 | -6.835 | -109.12 | -1.774 | -10.087 | 93.707 | -0.369 |
| 9 | -17.281 | -109.49 | -1.537 | -10.258 | 99.896 | 1.547 |
| 10 | -19.072 | -109.49 | -1.636 | -10.339 | 99.362 | 1.148 |
| 11 | -5.210 | -131.74 | -1.647 | -8.749 | 118.07 | 1.070 |
| 12 | -11.186 | -137.2 | -1.631 | -8.835 | 116.86 | 0.931 |
| r*1 | 0.396 | 0.247 | 0.730 | 0.250 | 0.432 | 0.789 |
| r*2 | 0.508 | 0.346 | 0.700 | 0.290 | 0.498 | 0.820 |
3. Experimental
3.1. Instruments, reagents and materials
3.2. Cell lines and cell cultures
3.3. Electrochemical measurements
3.4. Correlation coefficients
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds 1, 2 and 3 (mix), 5, 6, 9 and 11 are available from the authors.
References and Notes
- Asche, C. Antitumour quinones. Mini-Rev. Med. Chem. 2005, 5, 449–467. [Google Scholar] [CrossRef]
- Srinivas, G.; Babykutty, S.; Sathiadevan, P.P.; Srinivas, P. Molecular mechanism of emodin action: transition from laxative ingredient to an antitumor agent. Med. Res. Rev. 2007, 27, 591–608. [Google Scholar] [CrossRef]
- Foye, M.O. Cancer Chemotherapeutic Agents; American Chemical Society: Washigton, D.C., USA, 1995. [Google Scholar]
- Martinez, R.; Chacon-Garcia, L. The search of DNA-intercalators as antitumoral drugs: What it worked and what did not work. Curr. Med. Chem. 2005, 12, 127–151. [Google Scholar] [CrossRef]
- Rao, M.M.; Kingston, D.I. Plant anticancer agents. XII. Isolation and structure elucidation of new cytotoxic quinones from Tabebuia cassinoides. J. Nat. Prod. 1982, 45, 600–604. [Google Scholar] [CrossRef]
- Giorgi-Renault, S.G.; Renault, J.; Baron, M.; Servolles, P.; Paoletti, C.; Cros, S. Heterocyclic quinones. VI: Synthesis and antitumoral effects of 5,10-benzo[g]quinoxalinediones and aza-analogs. Eur. J. Med. Chem-Chim. Ther. 1985, 20, 144–148. [Google Scholar]
- Lee, H.-J.; Kim, J.S.; Park, S.-Y.; Suh, M.-E.; Kim, H.J.; Seo, E.-K.; Lee, C.-O. Synthesis and cytotoxicity evaluation of 6,11-dihydro-pyridazo- and 6,11-dihydro-pyrido[2,3-b]phenazine-6,11-diones. Bioorg. Med. Chem. 2004, 12, 1623–1628. [Google Scholar] [CrossRef]
- Pratt, Y.T.; Drake, N.L. Quinolinequinones. V. 6-Chloro- and 7-chloro-5,8-quinolinequinones. J. Am. Chem. Soc. 1960, 82, 1155–1161. [Google Scholar] [CrossRef]
- Katoh, A.; Ueda, S.; Ohkanda, J.; Hirota, M.; Komine, H.; Mitsuhashi, K. Isolation of the intermediates and improved synthesis of pyrido[1′,2′:1,2]imidazo[4,5-b]pyrazines and –quinoxalines. Heterocycles 1992, 34, 1965–1972. [Google Scholar] [CrossRef]
- Konoshima, T.; Kozuka, M.; Koyama, J.; Okatani, T.; Tagahara, K.; Tokuda, H. Studies on inhibitors of skin tumor promotion, VI. Inhibitory effects of quinones on Epstein-Barr virus activation. J. Nat. Prod. 1989, 52, 987–995. [Google Scholar] [CrossRef]
- Koyama, J.; Morita, I.; Tagahara, K.; Osakai, T.; Hotta, H.; Yang, M.X.; Mukainaka, T.; Nishino, H.; Tokuda, H. Correlation with redox potentials and inhibitory effects on Epstein-Barr virus activation of azaanthraquinones. Chem. Pharm. Bull. 2001, 49, 1214–1216. [Google Scholar] [CrossRef]
- Kano, K.; Konse, T.; Nishimura, N.; Kubota, T. Electrochemical properties of adriamycin absorbed on a mercury electrode surface. Bull. Chem. Soc. Jpn. 1984, 57, 2383–2390. [Google Scholar] [CrossRef]
- Koyama, J.; Tagahara, K.; Osakai, T.; Tsujino, Y.; Tsurumi, S.; Nishino, H.; Tokuda, H. Inhibitory effects on Epstein-Barr virus activation of anthraquinones: correlation with redox potentials. Cancer Lett. 1997, 115, 179–183. [Google Scholar] [CrossRef]
- Koyama, J.; Morita, I.; Kobayashi, N.; Osakai, T.; Hotta, H.; Takayasu, J.; Nishino, H.; Tokuda, H. Correlation of redox potentials and inhibitory effects on Epstein-Barr virus activation of naphthoquinones. Cancer Lett. 2003, 201, 25–30. [Google Scholar] [CrossRef]
- Koyama, J.; Morita, I.; Kobayashi, N.; Osakai, T.; Hotta, H.; Takayasu, J.; Nishino, H.; Tokuda, H. Correlation of redox potentials and inhibitory effects on Epstein-Barr virus activation of 2-azaanthraquinones. Cancer Lett. 2004, 212, 1–6. [Google Scholar] [CrossRef]
- Koyama, J.; Morita, I.; Kobayashi, N.; Osakai, T.; Nishino, H.; Tokuda, H. Correlation between reduction potentials and inhibitory effects on Epstein-Barr virus activation of poly-substituted anthraquinones. Cancer Lett. 2005, 225, 193–198. [Google Scholar] [CrossRef]
- Koyama, J.; Inoue, M.; Morita, I.; Kobayashi, N.; Osakai, T.; Nishino, H.; Tokuda, H. Correlation between reduction potentials and inhibitory effects on Epstein-Barr virus activation by emodin derivatives. Cancer Lett. 2006, 241, 263–267. [Google Scholar] [CrossRef]
- Koyama, J.; Nishino, Y.; Morita, I.; Kobayashi, N.; Osakai, T.; Tokuda, H. Correlation between reduction potentials and inhibitions of Epstein-Barr virus activation by anthraquinone derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 4106–4109. [Google Scholar] [CrossRef]
- Yamori, T.; Matsunaga, A.; Sato, S.; Yamazaki, K.; Komi, A.; Ishizu, K.; Mita, I.; Edatsugi, H.; Matsuba, Y.; Takezawa, K.; Nakanishi, O.; Kohno, H.; Nakajima, Y.; Komatsu, H.; Andoh, T.; Tsuruo, T. Quinolinequinones. V. 6-Chloro- and 7-chloro-5,8-quinolinequinones. Cancer Res. 1999, 59, 4042–4049. [Google Scholar]
- Dan, S.; Tsunoda, T.; Kitahara, O.; Yanagawa, R.; Zembutsu, H.; Katagiri, T.; Yamazaki, K.; Nakamura, Y.; Yamori, T. An integrated database of chemosensitivity to 55 anticancer drugs and gene expression profiles of 39 human cancer cell lines. Cancer Res. 2002, 62, 1139–1147. [Google Scholar]
- Yaguchi, S.; Fukui, Y.; Koshimizu, I.; Yoshimi, H.; Matsuno, T.; Gouda, H.; Hirono, S.; Yamazaki, K.; Yamori, T. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J. Natl. Cancer Inst. 2006, 98, 545–556. [Google Scholar] [CrossRef]
- Nakatsu, N.; Nakamura, T.; Yamazaki, K.; Sadahiro, S.; Makuuchi, H.; Kanno, J.; Yamori, T. Evaluation of action mechanisms of toxic chemicals using JFCR39, a panel of human cancer cell lines. Mol. Pharmacol. 2007, 72, 1171–1180. [Google Scholar] [CrossRef]
- Kong, D.; Dan, S.; Yamazaki, K.; Yamori, T. Inhibition profiles of phosphatidylinositol 3-kinase inhibitors against PI3K superfamily and human cancer cell line panel JFCR39. Eur. J. Cancer 2010, 46, 1111–1121. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods. I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Hirai, K.; Koyama, J.; Pan, J.; Simamura, E.; Shimada, H.; Yamori, T.; Sato, S.; Tagahara, K.; Tsuruo, T. Furanonaphthoquinone analogs possessing preferential in antitumor activity compared to normal cells. Cancer Detection Prev. 1999, 23, 539–550. [Google Scholar] [CrossRef]
- Watanabe, M.; Snieckus, V. Tandem directed metalation reactions. Short syntheses of polycyclic aromatic hydrocarbons and ellipticine alkaloids. J. Am. Chem. Soc. 1980, 102, 1457–1460. [Google Scholar] [CrossRef]
- Koyama, J.; Morita, I.; Kobayashi, N.; Osakai, T.; Usuki, Y.; Taniguchi, M. Structure-activity relations of azafluorenone and azaanthraquinone as antimicrobial compounds. Bioorg. Med. Chem Lett. 2005, 15, 1079–1082. [Google Scholar] [CrossRef]
- Paull, K.D.; Shoemaker, R.H.; Hodes, L.; Monks, A.; Scudiero, D.A.; Rubinstein, L.; Plowman, J.; Boyd, M.R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 1989, 81, 1088–1092. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.A.; Skehan, P.; Shoemaker, R.H.; Paull, K.D.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigo-Wolff, A.; Gary-Goodrichi, M.; Campbell, H.; Mayo, J.; Boyd, M.R. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Nat. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Yamori, T.; Sato, S.; Chikazawa, H.; Kadota, T. Anti-tumor efficacy of paclitaxel against human lung cancer xenografts. Jpn. J. Cancer Res. 1997, 88, 1205–1210. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Koyama, J.; Morita, I.; Yamori, T. Correlation between Cytotoxic Activities and Reduction Potentials of Heterocyclic Quinones. Molecules 2010, 15, 6559-6569. https://doi.org/10.3390/molecules15096559
Koyama J, Morita I, Yamori T. Correlation between Cytotoxic Activities and Reduction Potentials of Heterocyclic Quinones. Molecules. 2010; 15(9):6559-6569. https://doi.org/10.3390/molecules15096559
Chicago/Turabian StyleKoyama, Junko, Izumi Morita, and Takao Yamori. 2010. "Correlation between Cytotoxic Activities and Reduction Potentials of Heterocyclic Quinones" Molecules 15, no. 9: 6559-6569. https://doi.org/10.3390/molecules15096559
APA StyleKoyama, J., Morita, I., & Yamori, T. (2010). Correlation between Cytotoxic Activities and Reduction Potentials of Heterocyclic Quinones. Molecules, 15(9), 6559-6569. https://doi.org/10.3390/molecules15096559




