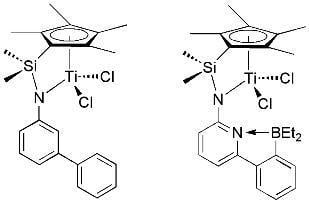
Homopolymerization of Ethylene, 1-Hexene, Styrene and Copolymerization of Styrene With 1,3-Cyclohexadiene Using (h5-Tetramethylcyclopentadienyl)dimethylsilyl(N-Ar’)amido-TiCl2/MAO (Ar’=6-(2-(Diethylboryl)phenyl)pyrid-2-yl, Biphen-3-yl)
Abstract
:1. Introduction



2. Results and Discussion
2.1. Synthesis and Homopolymerization of Ethylene
| # | Ti-8:MAO | T (°C) | Polymer yield (g) | Activity[a] | Mn (g/mol)[b] | PDI | Tm (°C) |
|---|---|---|---|---|---|---|---|
| 1 | 1:1000 | 50 | 1.03 | 110 | 1,800,000 | 1.02 | 131 |
| 2 | 1:1000 | 75 | 1.83 | 195 | 1,300,000 | 1.21 | 132 |
| # | Ti-3:MAO | T (°C) | Polymer yield (g) | Activity[a] | Mn (g/mol)[b] | PDI | Tm (°C) |
| 3 | 1:1000 | 50 | 2.87 | 270 | 1,500,000 | 1.14 | 134 |

2.3. Homopolymerization of 1-Hexene
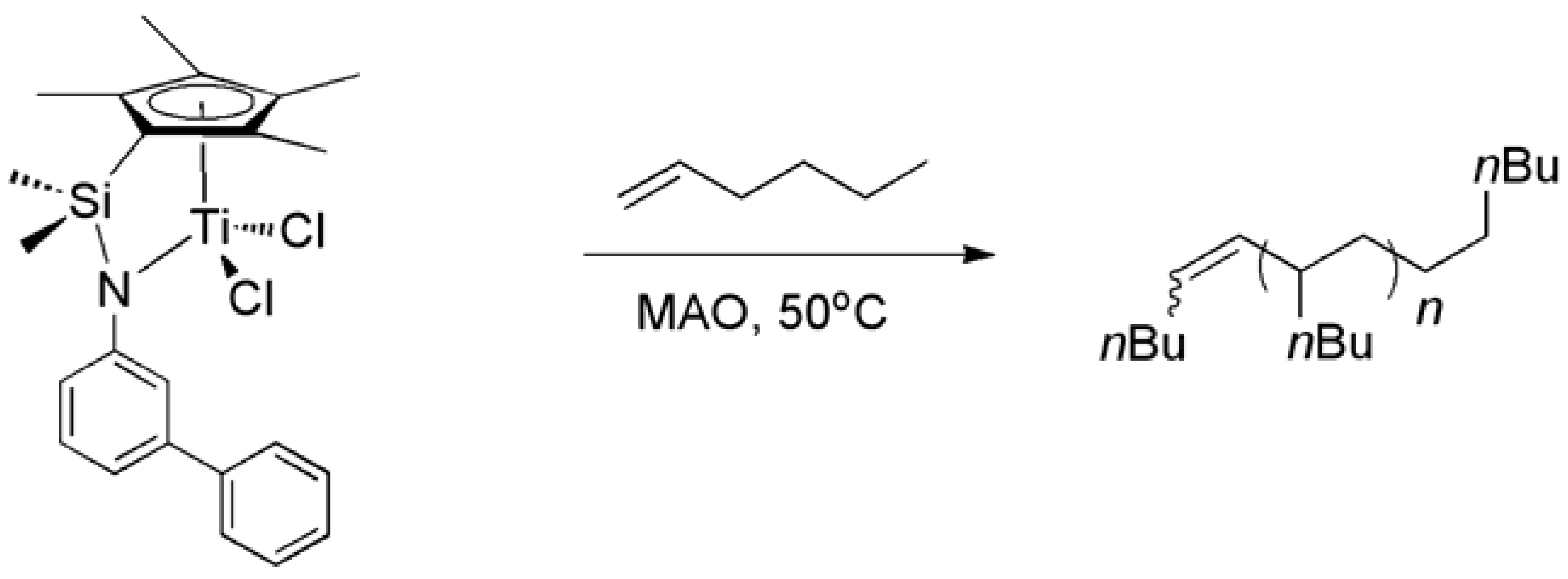
| Ti-3:MAO:1-hexene | Activity[a] | Polymer yield (g) | Mn [b] (g/mol) | PDI |
|---|---|---|---|---|
| 1:1,000:10,000 | 86 | 0.18 | 25,000 | 1.00 |


2.4. Homopolymerization of Styrene


| Cat:MAO:Styrene | T (°C) | Activity [a] | Yield (g) | Mn [b] (g/mol) | PDI | Tg/Tm (°C) | |
|---|---|---|---|---|---|---|---|
| Ti-8 | 1:2000:10000 | 25 | 285 | 0.54 | 91,000 | 1.92 | 96/272 |
| Ti-3 | 1:2000:10000 | 50 | 98 | 0.21 | 171,000 | 1.19 | 76/269 |
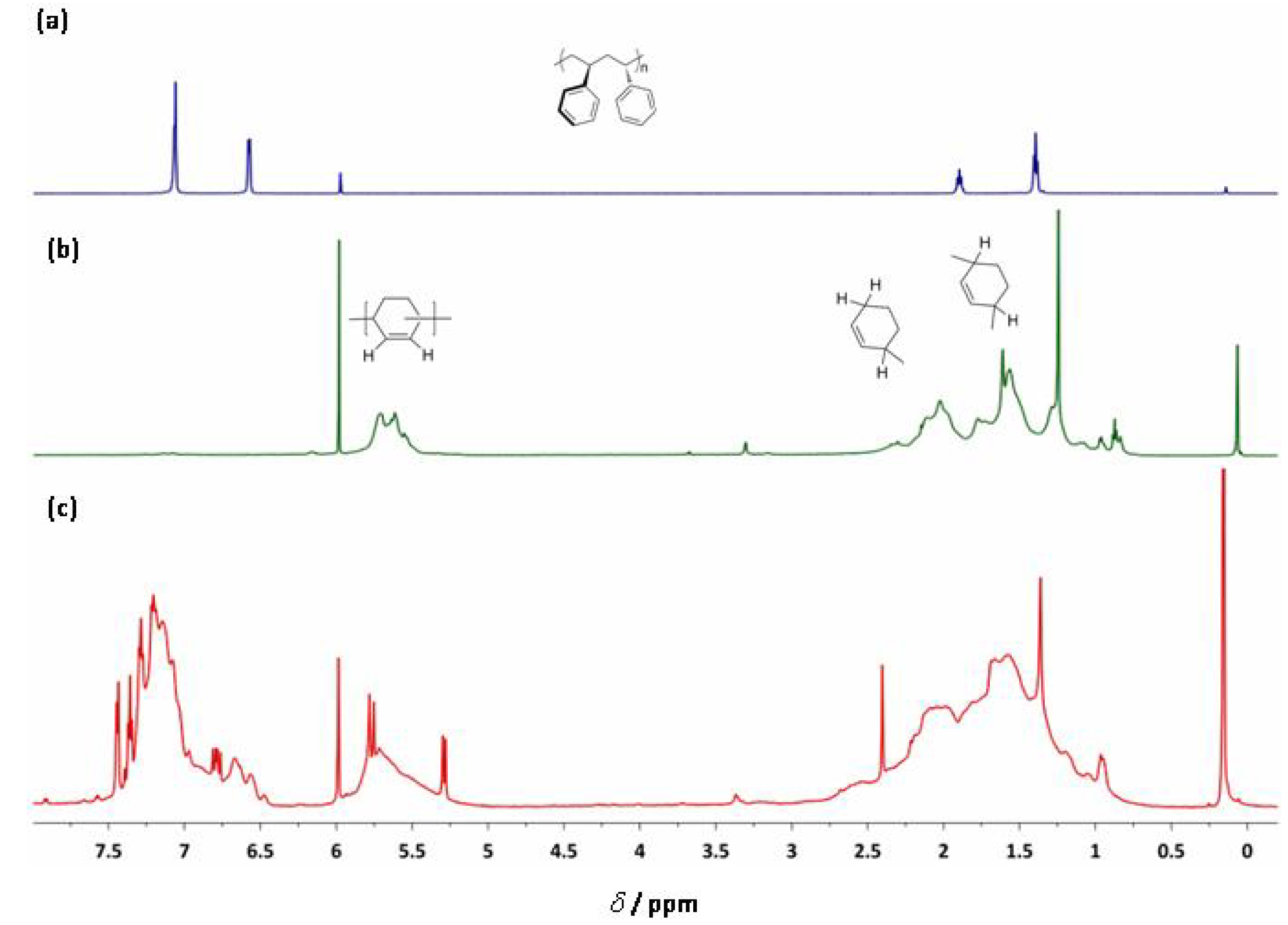
2.5. Homopolymerization of 1,3-Cyclohexadiene and its Copolymerization with Styrene
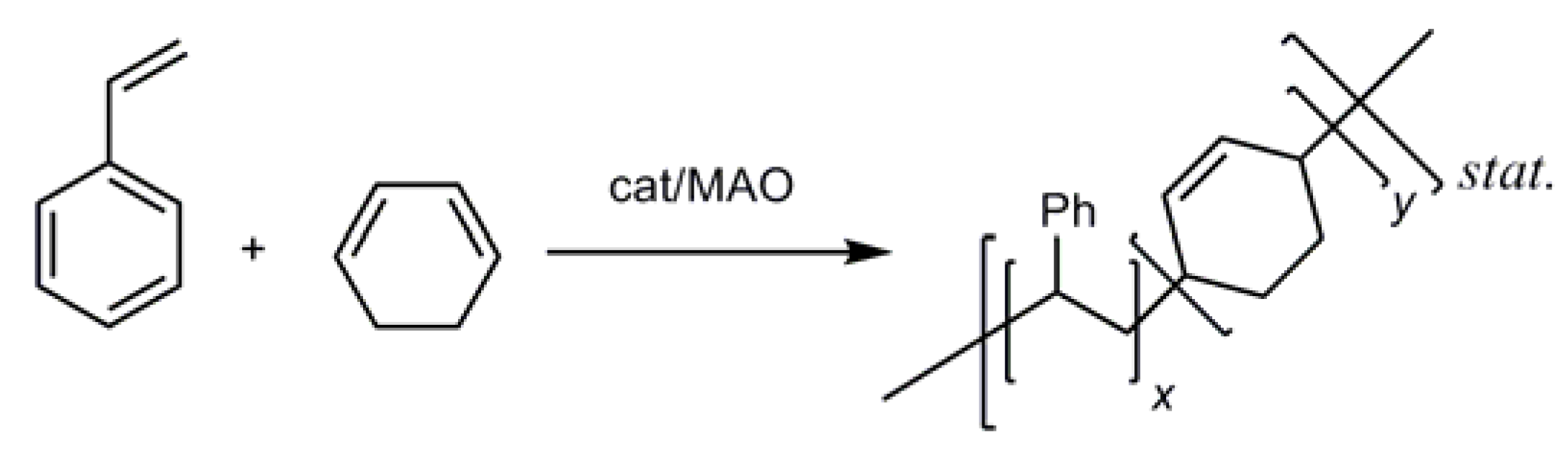
| Cat:MAO:Styrene:1,3-CHD | χ1,3-CHD | Activity[a] | Mn (g/mol) | PDI | Tg (oC) | Tm (oC) | |
|---|---|---|---|---|---|---|---|
| Ti-3 | 1:2,000:10,000:10,000 | 0 | 170 | 117,000 | 1.45 | 86 | 271 |
| Ti-8 | 1:2,000:10,000:10,000 | >20[b] | 640 | 288,000 | 1.89 | 103 | - |

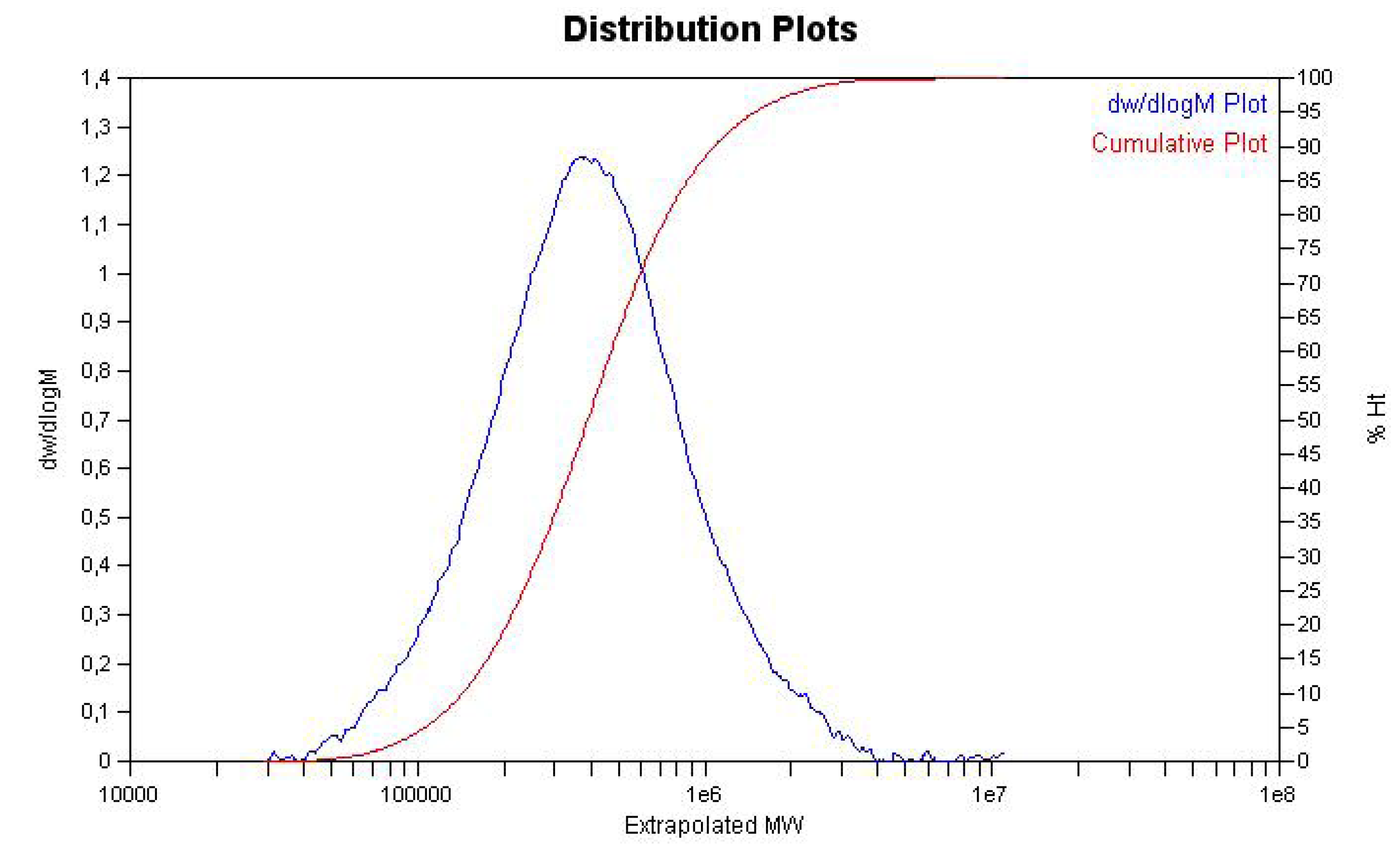
3. Experimental
3.1. General
3.2. Homopolymerization of Ethylene
3.3. Homopolymerization of Styrene and 1-Hexene
3.4. Copolymerization of Styrene with 1,3-Cyclohexadiene
4. Conclusions
Acknowledgements
References and Notes
- Hustad, P.D. Frontiers in Olefin Polymerization: Reinventing the World's Most Common Synthetic Polymers. Science 2009, 325, 704–707. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K. Advances in Non-Metallocene Olefin Polymerization Catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar]
- Sinn, H.; Kaminsky, W.; Vollmer, H.; Woldt, R. Living Polymers” on Polymerization with Extremely Productive Ziegler Catalysts. Angew. Chem. Int. Ed. 1980, 19, 390–392. [Google Scholar] [CrossRef]
- Sinn, H.; Kaminsky, W.; Stone, F.G.A.; Robert, W. Ziegler-Natta Catalysis. Adv. Organomet. Chem. 1980, 18, 99–149. [Google Scholar] [CrossRef]
- Kaminsky, W.; Miri, M.; Sinn, H.; Woldt, R. Bis(cyclopentadienyl)zirkon-verbindungen und Aluminoxan als Ziegler-Katalysatoren für die Polymerisation und Copolymerisation von Olefinen. Makromol. Chem. Rapid Commun. 1983, 4, 417–421. [Google Scholar] [CrossRef]
- Kaminsky, W.; Külper, K.; Brintzinger, H.H.; Wild, F.R.W.P. Polymerization of Propene and Butene with a Chiral Zirconocene and Methylalumoxane as Cocatalyst. Angew. Chem. Int. Ed. 1985, 24, 507–508. [Google Scholar] [CrossRef]
- Ostoja-Starzeski, K.A.; Kelly, W.M.; Stumpf, A.; Freitag, D. Donor/Acceptor Metallocenes: A New Structure Principle in Catalyst Design. Angew. Chem. Int. Ed. 1999, 38, 2439–2443. [Google Scholar] [CrossRef]
- Stevens, J.C.; Timmers, F.J.; Wilson, D.R.; Schmidt, G.F.; Nickias, P.N.; Rosen, R.K.; Knight, G.W.; Lai, S.-Y. Constrained Geometry Addition Polymerization Catalysts, Processes for Their Preparation, Precursors Therefor, Methods of Use and Novel Polymers Formed Therewi. Eur. Patent Appl. EP0416815, 1991. [Google Scholar]
- Canich, J.M.; Hlatky, G.; Turner, G.; Howard, W. Aluminum-free Monocyclopentadienyl Metallocene Catalysts for Olefin Polymerization. Int. Patent Appl. WO/1992/000333, 1992. [Google Scholar]
- McKnight, A.L.; Waymouth, R.M. Group 4 Ansa-Cyclopentadienyl-Amido Catalysts for Olefin Polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef]
- Braunschweig, H.; Breitling, F.M. Constrained Geometry Complexes--Synthesis and Applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Buchmeiser, M.R.; Camadanli, S.; Wang, D.; Zou, Y.; Decker, U.; Kühnel, C.; Reinhardt, I. A Tandem Catalyst for the Simultaneous Ring-Opening Metathesis / Vinyl Insertion Polymerization. 2010. submitted. [Google Scholar]
- Voskoboynikov, A.Z.; Ryabov, A.N.; Coker, C.L.; Canich, J.N.M. Process for Producing Substituted Metallocene Compounds for Olefin Polymerization. Int. Patent Appl. WO/2006/065844, 2006. [Google Scholar]
- Domski, G.J.; Rose, J.M.; Coates, G.W.; Bolig, A.D.; Brookhart, M. Living Alkene Polymerization: New Methods for the Precision Synthesis of Polyolefins. Prog. Polym. Sci. 2007, 32, 30–92. [Google Scholar] [CrossRef]
- Kleinschmidt, R.; Griebenow, Y.; Fink, G. Stereospecific Propylene Polymerization Using Half-Sandwich Metallocene/MAO Systems: A Mechanistic Insight. J. Mol. Cat. A Chem. 2000, 157, 83–90. [Google Scholar] [CrossRef]
- Axelson, D.E.; Levy, G.C.; Mandelkern, L. A Quantitative Analysis of Low-Density (Branched) Polyethylenes by Carbon-13 Fourier Transform Nuclear Magnetic Resonance at 67.9 MHz. Macromolecules 1979, 12, 41–52. [Google Scholar] [CrossRef]
- Galland, G.B.; de Souza, R.F.; Mauler, R.S.; Nunes, F.F. 13C NMR Determination of the Composition of Linear Low-Density Polyethylene Obtained with [η3-Methallyl-nickel-diimine]PF6 Complex. Macromolecules 1999, 32, 1620–1625. [Google Scholar] [CrossRef]
- Galland, G.B.; Quijada, R.; Rojas, R.; Bazan, G.; Komon, Z.J.A. NMR Study of Branched Polyethylenes Obtained with Combined Fe and Zr Catalysts. Macromolecules 2002, 35, 339–345. [Google Scholar] [CrossRef]
- Usami, T.; Takayama, S. Fine-Branching Structure in High-pressure, Low-density Polyethylenes by 50.10-MHz Carbon-13 NMR Analysis. Macromolecules 1984, 17, 1756–1761. [Google Scholar] [CrossRef]
- Malik, T.M.; Carreau, P.J.; Schreiber, H.P.; Rudin, A.; Kale, L.; Tchir, W. Property Stabilization in Filled, Drawn Polyethylene. J. Appl. Polym. Sci. 1991, 43, 543–551. [Google Scholar] [CrossRef]
- Liu, Z.; Somsook, E.; White, C.B.; Rosaaen, K.A.; Landis, C.R. Kinetics of Initiation, Propagation, and Termination for the [rac-(C2H4(1-indenyl)2)ZrMe][MeB(C6F5)3]-Catalyzed Polymerization of 1-Hexen. J. Am. Chem. Soc. 2001, 123, 11193–11207. [Google Scholar]
- Lamberti, M.; Pappalardo, D.; Zambelli, A.; Pellecchia, C. Syndiospecific Polymerization of Propene Promoted by Bis(salicylaldiminato)titanium Catalysts: Regiochemistry of Monomer Insertion and Polymerization Mechanism. Macromolecules 2002, 35, 658–663. [Google Scholar] [CrossRef]
- Mitani, M.; Furuyama, R.; Mohri, J.-i.; Saito, J.; Ishii, S.; Terao, H.; Nakano, T.; Tanaka, H.; Fujita, T. Syndiospecific Living Propylene Polymerization Catalyzed by Titanium Complexes Having Fluorine-Containing Phenoxy-Imine Chelate Ligands. J. Am. Chem. Soc. 2003, 125, 4293–4305. [Google Scholar]
- Lian, B.; Beckerle, K.; Spaniol, T.P.; Okuda, J. Regioselective 1-Hexene Oligomerization Using Cationic Bis(phenolato) Group-4 Metal Catalysts: Switch from 1,2- to 2,1-Insertion. Angew. Chem. Int. Ed. 2007, 46, 8507–8510. [Google Scholar] [CrossRef]
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific Olefin Polymerization with Chiral Metallocene Catalysts. Angew. Chem. Int. Ed. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- Ishihara, N.; Seimiya, T.; Kuramoto, M.; Uoi, M. Crystalline Syndiotactic Polystyrene. Macromolecules 1986, 19, 2464–2465. [Google Scholar] [CrossRef]
- Ishihara, N.; Kuramoto, M.; Uoi, M. Stereospecific Polymerization of Styrene Giving the Syndiotactic Polymer. Macromolecules 1988, 21, 3356–3360. [Google Scholar] [CrossRef]
- Malanga, M. Syndiotactic Polystyrene Materials. Adv. Mater. 2000, 12, 1869–1872. [Google Scholar] [CrossRef]
- Schellenberg, J. Recent Transition Metal Catalysts for Syndiotactic Polystyrene. Prog. Polym. Sci. 2009, 34, 688–718. [Google Scholar] [CrossRef]
- Rodrigues, A.-S.; Kirillov, E.; Lehmann, C.W.; Roisnel, T.; Vuillemin, B.; Razavi, A.; Carpentier, J.-F. Allyl Ansa-Lanthanidocenes: Single-Component, Single-Site Catalysts for Controlled Syndiospecific Styrene and Styrene–Ethylene (Co)Polymerization. Chem. Eur. J. 2007, 13, 5548–5565. [Google Scholar]
- Kirillov, E.; Lehmann, C.W.; Razavi, A.; Carpentier, J.-F. Highly Syndiospecific Polymerization of Styrene Catalyzed by Allyl Lanthanide Complexes. J. Am. Chem. Soc. 2004, 126, 12240–12241. [Google Scholar]
- Jensen, T.R.; Yoon, S.C.; Dash, A.K.; Luo, L.; Marks, T.J. Organotitanium-Mediated Stereoselective Coordinative/Insertive Homopolymerizations and Copolymerizations of Styrene and Methyl Methacrylate. J. Am. Chem. Soc. 2003, 125, 14482–14494. [Google Scholar] [CrossRef]
- Feil, F.; Harder, S. New Stereochemical Assignments of 13C NMR Signals for Predominantly Syndiotactic Polystyrene. Macromolecules 2003, 36, 3446–3448. [Google Scholar] [CrossRef]
- Tonelli, A.E. Stereosequence-dependent Carbon-13 NMR Chemical Shifts in Polystyrene. Macromolecules 1983, 16, 604–607. [Google Scholar] [CrossRef]
- Rodrigues, A.-S.; Kirillov, E.; Carpentier, J.-F. Group 3 and 4 Single-Site Catalysts for Stereospecific Polymerization of Styrene. Coord. Chem. Rev. 2008, 252, 2115–2136. [Google Scholar] [CrossRef]
- Hong, B.K.; Jo, W.H.; Kim, J. Miscibility of Syndiotactic Polystyrene/Atactic Polystyrene Blends by Crystallization Kinetics and Enthalpy Relaxation. Polymer 1998, 39, 3753–3757. [Google Scholar] [CrossRef]
- Chen, C.-M.; Hsieh, T.-E.; Ju, M.-Y. Effects of Polydispersity Index and Molecular Weight on Crystallization Kinetics of Syndiotactic Polystyrene (sPS). J. Alloys Compd. 2009, 480, 658–661. [Google Scholar] [CrossRef]
- Williamson, D.T.; Elman, J.F.; Madison, P.H.; Pasquale, A.J.; Long, T.E. Synthesis and Characterization of Poly(1,3-cyclohexadiene) Homopolymers and Star-Shaped Polymers. Macromolecules 2001, 34, 2108–2114. [Google Scholar] [CrossRef]
- Longo, P.; Freda, C.; Ruiz de Ballesteros, O.; Grisi, F. Highly Stereoregular Polymerization of 1,3-Cyclohexadiene in the Presence of Cp2Ni-MAO Catalyst. Macromol. Chem. Phys. 2001, 202, 409–412. [Google Scholar] [CrossRef]
- Sharaby, Z.; Martan, M.; Jagur-Grodzinski, J. Stereochemistry of Poly(1,3-cyclohexadienes). NMR Investigation of Effects due to the Solvent Medium and to the Mechanism of Polymerization. Macromolecules 1982, 15, 1167–1173. [Google Scholar] [CrossRef]
- Hong, K.; Mays, J.W. 1,3-Cyclohexadiene Polymers. 1. Anionic Polymerization. Macromolecules 2001, 34, 782–786. [Google Scholar] [CrossRef]
- Williamson, D.T.; Buchanan, T.D.; Elkins, C.L.; Long, T.E. Synthesis of Block Copolymers Based on the Alternating Anionic Copolymerization of Styrene and 1,3-Cyclohexadiene. Macromolecules 2004, 37, 4505–4511. [Google Scholar] [CrossRef]
- Imaizumi, K.; Ono, T.; Natori, I.; Sakurai, S.; Takeda, K. Microphase-Separated Structure of 1,3-Cyclohexadiene/butadiene Triblock Copolymers and Its effect on Mechanical and Thermal Properties. J. Polym. Sci, Part B: Polym. Phys. 2001, 39, 13–22. [Google Scholar] [CrossRef]
- Natori, I.; Inoue, S. Living Anionic Polymerization of 1,3-Cyclohexadiene with the n-Butyllithium/N,N,N',N'-Tetramethylethylenediamine System. Copolymerization and Block Copolymerization with Styrene, Butadiene, and Isoprene. Macromolecules 1998, 31, 982–987. [Google Scholar] [CrossRef]
- Hong, K.; Mays, J.W. 1,3-Cyclohexadiene Polymers. 3. Synthesis and Characterization of Poly(1,3-cyclohexadiene-block-styrene). Macromolecules 2001, 34, 3540–3547. [Google Scholar] [CrossRef]
- Heiser, D.E.; Okuda, J.; Gambarotta, S.; Mülhaupt, R. Catalytic 1,3-Cyclohadiene Homopolymerization and Regioselective Copolymerization with Ethylene. Macromol. Rapid Commun. 2005, 206, 195–202. [Google Scholar] [CrossRef]
- Natori, I.; Imaizumi, K.; Yamagishi, H.; Kazunori, M. Hydrocarbon Polymers Containing Six-membered Rings in the Main Chain. Microstructure and Properties of Poly(1,3-cyclohexadiene). J. Polym. Sci., Part B: Polym. Phys. 1998, 36, 1657–1668. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Camadanli, S.; Decker, U.; Kühnel, C.; Reinhardt, I.; Buchmeiser, M.R. Homopolymerization of Ethylene, 1-Hexene, Styrene and Copolymerization of Styrene With 1,3-Cyclohexadiene Using (h5-Tetramethylcyclopentadienyl)dimethylsilyl(N-Ar’)amido-TiCl2/MAO (Ar’=6-(2-(Diethylboryl)phenyl)pyrid-2-yl, Biphen-3-yl). Molecules 2011, 16, 567-582. https://doi.org/10.3390/molecules16010567
Camadanli S, Decker U, Kühnel C, Reinhardt I, Buchmeiser MR. Homopolymerization of Ethylene, 1-Hexene, Styrene and Copolymerization of Styrene With 1,3-Cyclohexadiene Using (h5-Tetramethylcyclopentadienyl)dimethylsilyl(N-Ar’)amido-TiCl2/MAO (Ar’=6-(2-(Diethylboryl)phenyl)pyrid-2-yl, Biphen-3-yl). Molecules. 2011; 16(1):567-582. https://doi.org/10.3390/molecules16010567
Chicago/Turabian StyleCamadanli, Sebnem, Ulrich Decker, Christa Kühnel, Ingrid Reinhardt, and Michael R. Buchmeiser. 2011. "Homopolymerization of Ethylene, 1-Hexene, Styrene and Copolymerization of Styrene With 1,3-Cyclohexadiene Using (h5-Tetramethylcyclopentadienyl)dimethylsilyl(N-Ar’)amido-TiCl2/MAO (Ar’=6-(2-(Diethylboryl)phenyl)pyrid-2-yl, Biphen-3-yl)" Molecules 16, no. 1: 567-582. https://doi.org/10.3390/molecules16010567