Components of Rhizome Extract of Cnidium officinale Makino and Their In vitro Biological Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Compounds
2.1.1. Falcarindiol

2.1.2. (Z)-6-Hydroxy-7-methoxy-dihydroligustilide
2.1.3. Ligustilidiol and Senkyunolide H
2.2. Measurement of Anti-Inflammation Activity
2.2.1. Assessment of Cell Cytotoxicity
2.2.2. Effects of Compounds on NO Production in LPS-Stimulated RAW 264.7 Cells


| Treatment | IC50 (µM) * |
|---|---|
| Falcarindiol | 4.31 ± 5.22 |
| 6-hydroxy-7-methoxy-dihydroligustilide | 152.95 ± 4.23 |
| Ligustilidiol | 72.78 ±5.13 |
| Senkyunolide H | 173.42 ± 3.22 |
| NIL | 8.70 ± 2.34 |
2.2.3. RT-PCR for Anti-Inflammatory Effects
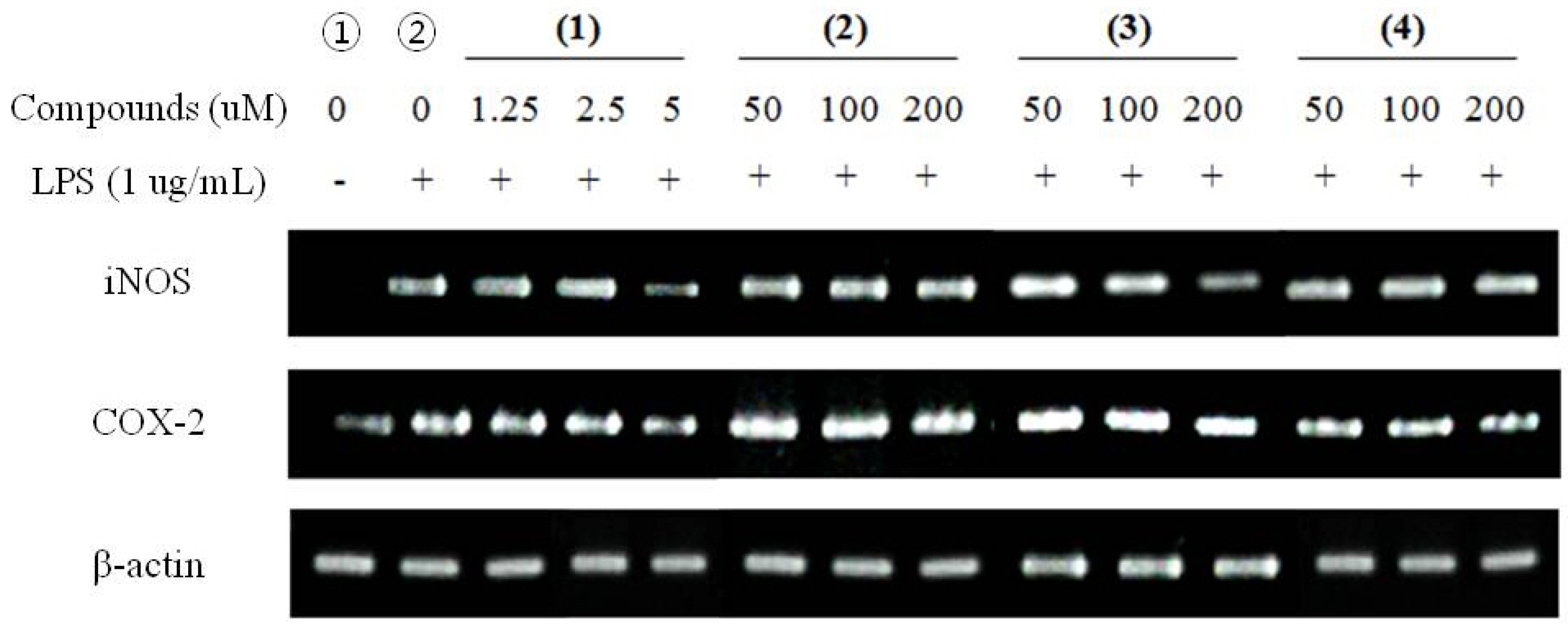
2.3. Measurement of Anti-Cancer Activity
2.3.1. Cytotoxic Effects of Falcarindiol

2.3.2. Cell Cycle Assay by Flow Cytometry

2.3.3. Observation of Morphological Change

2.3.4. RT-PCR for Anti-Cancer Effects

3. Experimental Section
3.1. Extraction and Isolation
3.1.1. Extraction and Partition
3.1.2. Isolation of Compounds
3.1.3. Identification of Compounds
3.2. Cell Cultures
3.3. Assessment of Cell Viability
3.4. Effects of Compounds on NO Production in LPS-Stimulated RAW 264.7 Cells
3.5. Measurement of Anti-Cancer Activity
3.5.1. Cell Cycle Assay by Flow Cytometry
3.5.2. Observation of Morphological Changes
3.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
| Genes | Primer | Sequence |
|---|---|---|
| iNOS | Sense | ATTGGCAACATCAGGTCGGCCATCACT |
| Antisense | GCTGTGTGTCACAGAAGTCTCGAAGTC | |
| COX-2 | Sense | GGAGAGACTATCAAGATAGT |
| Antisense | ATGGTGAGTAGACTTTTACA | |
| Bcl-2 | Sense | AGCTGCACCTGACGCCCTTCA |
| Antisense | AGCCAGGAGAAATCACAGAGG | |
| Bax | Sense | ATGGACGGGT CCGGGGAGCAG |
| Antisense | CAGTTGAAGTTGCCGTCAGA | |
| p53 | Sense | GGGACAGCCAAGTCTGTG |
| Antisense | GGAGTCTTCCA GTGTGAT | |
| β-actin (anti-inflammatory) | Sense | TCATGAAGTGTGACGTTGACATCCGT |
| Antisense | CCTAGAAGCATTTGCGGTTCACGATG | |
| β-actin (anti-cancer) | Sense | CCTCTATGC CAACACAGTGC |
| Antisense | ATACTCCTGCTTGCTGATCC |
4. Conclusions
Acknowledgments
References and Notes
- Park, J.H.; Lee, J.G. The Encyclopedia of Medicinal Plants; Sin-il: Seoul, Korea, 2000; p. 418. [Google Scholar]
- Kim, M.S.L.; Choi, H.S.; Sawamura, M. Constituents of the essential oil of cnidium officinale Makino, a Korean medicinal plant. Flavour. Frag. J. 2002, 17, 49–53. [Google Scholar] [CrossRef]
- Tahara, E.; Satoh, T.; Toriizuka, K.; Nagai, H.; Nunome, S.; Shimada, Y.; Itoh, T.; Terasawa, K.; Saiki, I. Effect of Shimotsu-to (a Kampo medicine, Si-Wu-Tang) and its constituents on triphasic skin reaction in passively sensitized mice. J. Ethnopharmacol. 1999, 68, 219–228. [Google Scholar] [CrossRef]
- Wang, J.D.; Narui, T.; Kurata, H.; Takeuchi, K.; Hashimoto, T.; Okuyama, T. Hematological studies on naturally occurring substances. II. Effects of animal crude drugs on blood coagulation and fibrinolysis systems. Chem. Pharm. Bull. (Tokyo) 1989, 37, 2236–2238. [Google Scholar] [CrossRef]
- Higashi, K. The therapeutic effect of Unsei-in on facial redness (inflammatory congestion) in atopic dermatitis. Jpn. J. Oriental Med. 1996, 46, 753–760. [Google Scholar]
- Haranaka, K.; Satomi, N.; Sakurai, A.; Haranaka, R.; Okada, N.; Kobayashi, M. Antitumor activities and tumor necrosis factor producibility of traditional Chinese medicines and crude drugs. Cancer Immun. Immunother. 1985, 20, 1–5. [Google Scholar]
- Onishi, Y.; Yamaura, T.; Tauchi, K.; Sakamoto, T.; Tsukada, K.; Nunome, S.; Komatsu, Y.; Saiki, I. Expression of the anti-metastatic effect induced by Juzen-taiho-to is based on the content of Shimotsu-to constituents. Biol. Pharm. Bull. 1998, 21, 761–5. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Meth. Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar] [CrossRef]
- Beal, M.F. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 1995, 38, 357–366. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Leung, P.S.; Chan, Y.C. Role of oxidative stress in pancreatic inflammation. Antioxid. Redox Signal. 2009, 11, 135–165. [Google Scholar] [CrossRef]
- Lawless, M.W.; O'Byrne, K.J.; Gray, S.G. Oxidative stress induced lung cancer and COPD: opportunities for epigenetic therapy. J. Cell. Mol. Med. 2009, 13, 2800–2821. [Google Scholar] [CrossRef]
- Goldstein, M.G.; Li, Z. Heat-shock proteins in infection-mediated inflammation-induced tumorigenesis. J. Hematol. Oncol. 2009, 2, 5. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.J. Inflammation: Gearing the journey to cancer. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef]
- Reddy, L.; Odhav, B.; Bhoola, K.D. Natural products for cancer prevention: A global perspective. Pharmacol. Ther. 2003, 99, 1–13. [Google Scholar] [CrossRef]
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer 2002, 2, 143–148. [Google Scholar] [CrossRef]
- Deng, S. Phytochemical investigation of bioactive constituents from angelica sinesis. Ph.D. Thesis, University of Illinois, Chicago, IL, USA, 2005. [Google Scholar]
- Grech, J.N.; Li, Q.; Roufogalis, B.D.; Duck, C.C. Novel Ca(2+)-ATPase inhibitors from the dried root tubers of Polygonum Multiflorum. J. Nat. Prod. 1994, 57, 1682–1687. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fujita, M.; Mitsuhashi, H. Components of Cnidium officinale Makino: Occurrence of pregnenolone, coniferyl ferulate, and hydroxyphthalides. Chem. Pharm. Bull. (Tokyo) 1984, 32, 3770–3773. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar]
- Lee, K.W.; Kim, H.J.; Lee, Y.S.; Park, H.J.; Choi, J.W.; Ha, J.; Lee, K.T. Acteoside inhibits human promyelocytic HL-60 leukemia cell proliferation via inducing cell cycle arrest at G0/G1 phase and differentiation into monocyte. Carcinogenesis 2007, 28, 1928–1936. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bae, K.-E.; Choi, Y.-W.; Kim, S.-T.; Kim, Y.-K. Components of Rhizome Extract of Cnidium officinale Makino and Their In vitro Biological Effects. Molecules 2011, 16, 8833-8847. https://doi.org/10.3390/molecules16108833
Bae K-E, Choi Y-W, Kim S-T, Kim Y-K. Components of Rhizome Extract of Cnidium officinale Makino and Their In vitro Biological Effects. Molecules. 2011; 16(10):8833-8847. https://doi.org/10.3390/molecules16108833
Chicago/Turabian StyleBae, Ki-Eun, Young-Woong Choi, Sang-Tae Kim, and Young-Kyoon Kim. 2011. "Components of Rhizome Extract of Cnidium officinale Makino and Their In vitro Biological Effects" Molecules 16, no. 10: 8833-8847. https://doi.org/10.3390/molecules16108833




