Phenolic Compounds from Allium schoenoprasum, Tragopogon pratensis and Rumex acetosa and Their Antiproliferative Effects
Abstract
:1. Introduction
2. Results and Discussion
| PC | Allium schoenoprasum | Tragopogon pratensis | Rumex acetosa | ||||
|---|---|---|---|---|---|---|---|
| Extract | Dry matter | Extract | Dry matter | Extract | Dry matter | ||
| (µg/mL) | (µg/g) | (µg/mL) | (µg/g) | (µg/mL) | (µg/g) | ||
| GA | Gallic acid | 8.45 | 201.76 | 66.16 | 1347.85 | / | / |
| CA | Coumaric acid | 8.50 | 207.29 | / | / | / | / |
| FA | Ferulic acid | 37.16 | 887.44 | 9.71 | 197.79 | / | / |
| Ru | Rutin | 0.85 | 20.26 | 4.39 | 89.99 | / | / |
| Re | Resveratrol | / | / | 0.68 | 13.95 | 3.23 | 41.27 |
| VA | Vanillic acid | / | / | / | / | 11.03 | 130.29 |
| SA | Sinapic acid | / | / | 5.28 | 110.85 | 483.21 | 5708.48 |
| C | Catechin | / | / | / | / | 6.39 | 75.46 |
| CA | Caffeic acid | / | / | 13.68 | 278.72 | / | / |
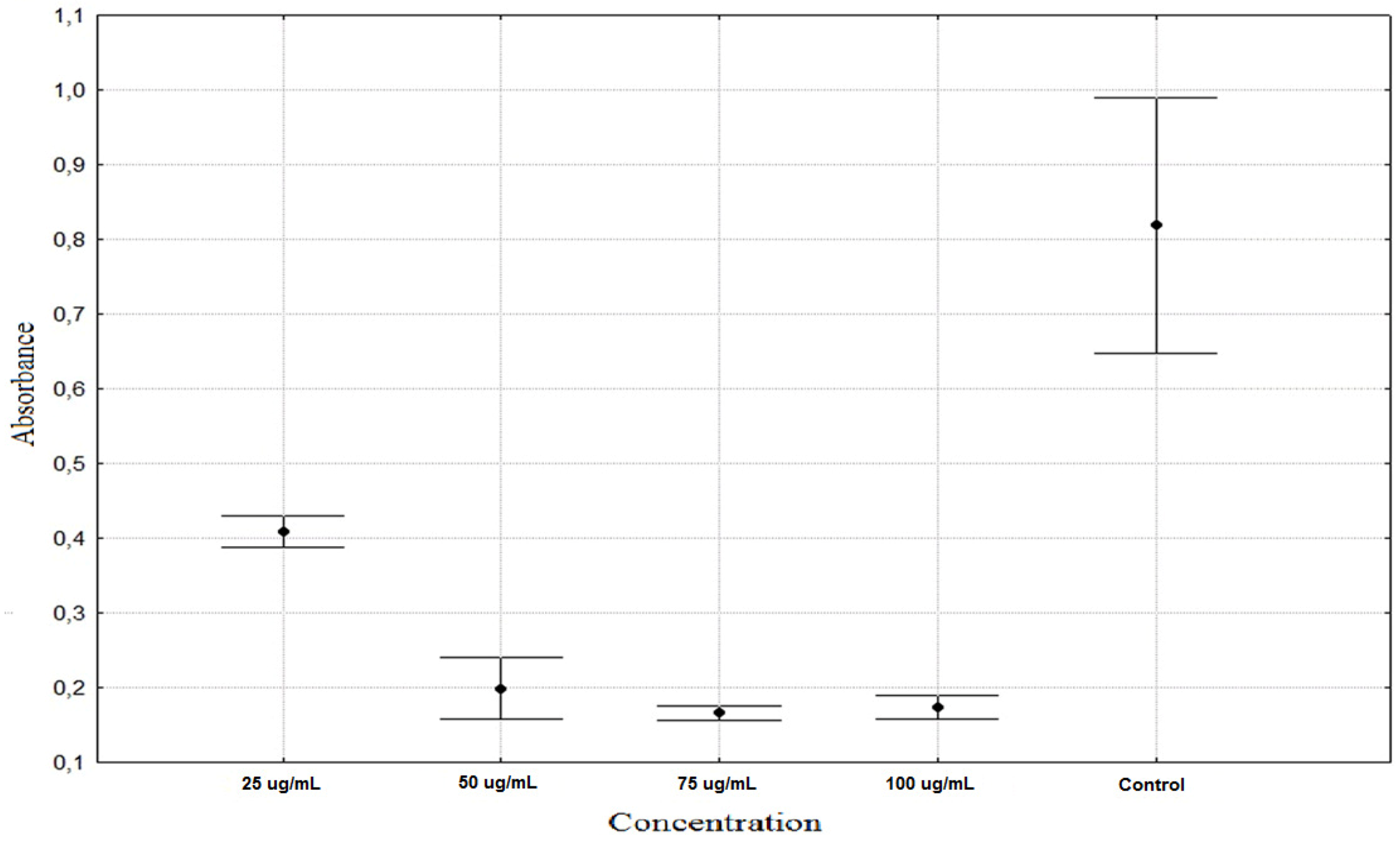
| Allium schoenoprasum 25 µg/mL | 0.1975 ± 0.0128 ** |
| Allium schoenoprasum 50 µg/mL | 0.2043 ± 0.0253 ** |
| Allium schoenoprasum 75 µg/mL | 0.2151 ± 0.0164 ** |
| Allium schoenoprasum 100 µg/mL | 0.1930 ± 0.0221 ** |
| Rumex acetosa 25 µg/mL | 0.5873 ± 0.0671 ** |
| Rumex acetosa 50 µg/mL | 0.4472 ± 0.0643 ** |
| Rumex acetosa 75 µg/mL | 0.2367 ± 0.0578 ** |
| Rumex acetosa 100 µg/mL | 0.1903 ± 0.0203 ** |
| Tragopogon pratensis 25 µg/mL | 0.4090 ± 0.0216 ** |
| Tragopogon pratensis 50 µg/mL | 0.1991 ± 0.0433 ** |
| Tragopogon pratensis 75 µg/mL | 0.1666 ± 0.0104 ** |
| Tragopogon pratensis 100 µg/mL | 0.1738 ± 0.0165 ** |
| Control | 0.8187 ± 0.1806 * |



3. Experimental
3.1. Extraction Conditions
3.2. Cell Cultivation
3.3. Antiproliferation Test
3.4. Determination of PhC
3.5. Chromatography
4. Conclusions
Acknowledgments
References
- Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Protective effects of green tea polyphenols administered by oral intubation against chemical carcinogen-induced forestomach and pulmonary neoplasia in A/J mice. Cancer Lett. 1993, 73, 167–172. [Google Scholar] [CrossRef]
- Sharif, T.; Auger, C.; Alhosin, M.; Ebel, C.; Achour, M.; Etienne-Selloum, N.; Fuhrmann, G.; Bronner, C.; Schini-Kerth, V.B. Red wine polyphenols cause growth inhibition and apoptosis in acute lymphoblastic leukaemia cells by inducing a redoxsensitive up-regulation of p73 and down-regulation of UHRF1. Eur. J. Cancer 2010, 46, 983–994. [Google Scholar] [CrossRef]
- Luceri, C.; Caderni, G.; Sanna, A.; Dolara, P. Red Wine and Black Tea Polyphenols Modulate the Expression of Cycloxygenase-2, Inducible Nitric Oxide Synthase and Glutathione-Related Enzymes in Azoxymethane-Induced F344 Rat Colon Tumors. J. Nutr. 2002, 132, 1376–1379. [Google Scholar]
- Iwasawa, H.; Morita, E.; Yui, S.; Yamazaki, M. Anti-oxidant Effects of Kiwi Fruit in Vitro and in Vivo. Biol. Pharm. Bull. 2011, 34, 128–134. [Google Scholar] [CrossRef]
- Rop, O.; Sochor, J.; Jurikova, T.; Zitka, O.; Skutkova, H.; Mlcek, J.; Salas, P.; Krska, B.; Babula, P.; Adam, V.; Kramarova, D.; Beklova, M.; Provaznik, I.; Kizek, R. Effect of five different stages of ripening on chemical compounds in medlar (Mespilus germanica L.). Molecules 2011, 16, 74–91. [Google Scholar]
- Kuroda, Y.; Hara, Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat. Res. 1999, 436, 69–97. [Google Scholar] [CrossRef]
- Castillo-Pichardo, L.; Martínez-Montemayor, M.M.; Martínez, J.E.; Wall, K.M.; Cubano, L.A.; Dharmawardhane, S. Inhibition of mammary tumor growth and metastases to bone and liver by dietary grape polyphenols. Clin. Exp. Metastasis 2009, 26, 505–516. [Google Scholar] [CrossRef]
- Jin, H.; Tan, X.; Liu, X.; Ding, Y. The Study of Effect of Tea Polyphenols on Microsatellite Instability Colorectal Cancer and Its Molecular Mechanism. Int. J. Colorectal Dis. 2010, 25, 1407–1415. [Google Scholar] [CrossRef]
- Mlček, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Tech. 2011. In Press. [Google Scholar]
- Rop, O.; Mlček, J.; Juríková, T.; Valšíková, M.; Sochor, J.; Reznicek, V.; Kramarova, D. Phenolic content, antioxidant capacity, radical oxygen species scavenging and lipid peroxidation inhibiting activities of extracts of five black chokeberry (Aronia melanocarpa (Michx.) Elliot) cultivars. J. Med. Plants Res. 2010, 4, 2431–2437. [Google Scholar]
- Walter, A.; Etienne-Selloum, N.; Sarr, M.; Kane, M.O.; Beretz, A.; Schini-Kerth, V.B. Angiotensin II induces the vascular expression of VEGF and MMP-2 in vivo: Preventive effect of red wine polyphenols. J. Vasc. Res. 2008, 45, 386–394. [Google Scholar] [CrossRef]
- Schlachterman, A.; Valle, F.; Wall, K.M.; Azios, N.G.; Castillo, L.; Morell, L.; Washington, A.V.; Cubano, L.A.; Dharmawardhane, S.F. Combined Resveratrol, Quercetin, and Catechin Treatment Reduces Breast Tumor Growth in a Nude Mouse Model. Transl. Oncol. 2008, 1, 19–27. [Google Scholar]
- Oak, M.H.; El Bedoui, J.; Schini-Kerth, V.B. Antiangiogenic properties of natural polyphenols from red wine and green tea. J. Nutr. Biochem. 2005, 16, 1–8. [Google Scholar] [CrossRef]
- Harris, D.M.; Besselink, E.; Henning, S.M.; Go, V.L.; Heber, D. Phytoestrogens induce differential estrogen receptor alpha- or beta-mediated responses in transfected breast cancer cells. Exp. Biol. Med. 2005, 230, 558–568. [Google Scholar]
- Roussou, I.; Lambropoulos, I.; Pagoulatos, G.N.; Roussis, I.G. Decrease of heat shock protein levels in hela tumor cells by red wine extracts. Ital. J. Food Sci. 2004, 16, 381–386. [Google Scholar]
- Lin, J.K.; Liang, Y.C.; Lin-Shiau, S.Y. Cancer Chemoprevention by Tea Polyphenols through Mitotic Signal Transduction Blockade. Biochem. Pharmacol. 1999, 58, 911–915. [Google Scholar] [CrossRef]
- Soleas, G.J.; Grass, L.; Josephy, P.D.; Goldberg, D.M.; Diamandis, E.P. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin. Biochem. 2002, 35, 119–124. [Google Scholar] [CrossRef]
- Nichenametla, S.N.; Taruscio, T.G.; Barney, D.L.; Exon, J.H. A Review of the Effects and Mechanisms of Polyphenolics in Cancer. Crit. Rev. Food Sci. 2006, 46, 161–183. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef]
- Navarro-Perán, E.; Cabezas-Herrera, J.; Campo, L.S.; Rodríguez-López, J.N. Effects of folate cycle disruption by the green tea polyphenol epigallocatechin-3-gallate. Int. J. Biochem. Cell Biol. 2007, 39, 2215–2225. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food. Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of PhC in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52. [Google Scholar] [CrossRef]
- Proestos, C.; Kapsokefalou, M.; Komaitis, M. Analysis of naturally occurring phenolic compounds in aromatic plants by RP-HPLC and GC-MS after silylation. J. Food Qual. 2008, 31, 402–414. [Google Scholar] [CrossRef]
- Lin, X.F.; Min, W.; Luo, D. Anticarcinogenic effect of ferulic acid on ultraviolet-B irradiated human keratinocyte HaCaT cells. J. Med. Plants Res. 2010, 4, 1686–1694. [Google Scholar]
- Baskaran, N.; Manoharan, S.; Balakrishnan, S.; Pugalendhi, P. Chemopreventive potential of ferulic acid in 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis in Sprague—Dawley rats. Eur. J. Pharmacol. 2010, 637, 22–29. [Google Scholar] [CrossRef]
- Salucci, M.; Stivala, L.A.; Maiani, G.; Bugianesi, R.; Vannini, V. Flavonoids uptake and their effect on cell cycle of human colon adenocarcinoma cells (Caco2). Br. J. Cancer 2002, 86, 1645–1651. [Google Scholar] [CrossRef]
- Sohi, K.K.; Mittal, N.; Hundal, M.K.; Khanduja, K.L. Gallic acid, an antioxidant, exhibits anti apoptotic potential in normal human lymphocytes: a Bcl-2 independent mechanism. J. Nutr. Sci. Vitaminol. 2003, 49, 221–227. [Google Scholar] [CrossRef]
- Kampa, M.; Alexaki, V.I.; Notas, G.; Nifli, A.P.; Nistikaki, A.; Hatzoglou, A.; Bakogeorgou, B.; Kouimtzoglou, E.; Blekas, G.; Boskou, D.; Gravanis, A.; Castanas, E. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004, 6, 63–74. [Google Scholar]
- Murugan, R.S.; Priyadarsini, R.V.; Ramalingam, K.; Hara, Y.; Karunagaran, D.; Nagini, S. Intrinsic apoptosis and NF-κB signaling are potential molecular targets for chemoprevention by black tea polyphenols in HepG2 cells in vitro and in a rat hepatocarcinogenesis model in vivo. Food Chem. Toxicol. 2010, 48, 3281–3287. [Google Scholar] [CrossRef]
- Way, T.D.; Lin, H.Y.; Hua, K.T.; Lee, J.C.; Li, W.H.; Lee, M.R.; Shuang, C.H.; Lin, J.K. Beneficial effects of different tea flowers against human breast cancer MCF-7 cells. Food Chem. 2009, 114, 1231–1236. [Google Scholar] [CrossRef]
- Lin, J.K. Cancer Chemoprevention by Tea Polyphenols through Modulating Signal Transduction Pathways. Arch. Pharm. Res. 2002, 25, 561–571. [Google Scholar] [CrossRef]
- Filomeni, G.; Graziani, I.; Rotilio, G.; Ciriolo, M.R. trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr. 2007, 2, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.T.; Yen, G.C. Involvement of p38 MAPK and Nrf2 in phenolic acid-induced P-form phenol sulfotransferase expression in human hepatoma HepG2 cells. Carcinogenesis 2006, 27, 1008–1017. [Google Scholar]
- Ma, Z.C.; Hong, Q.; Wang, Y.G.; Tan, H.L.; Xiao, C.R.; Liang, Q.D.; Zhang, B.L.; Gao, Y. Ferulic acid protects human umbilical vein endothelial cells from radiation induced oxidative stress by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Biol. Pharm. Bull. 2010, 33, 29–34. [Google Scholar] [CrossRef]
- Maggi-Capeyron, M.F.; Ceballos, P.; Cristol, J.P.; Delbosc, S.; Le Doucen, C.; Pons, M.; Léger, C.L.; Descomps, B. Wine phenolic antioxidants inhibit AP-1 transcriptional activity. J. Agric. Food Chem. 2001, 49, 5646–5652. [Google Scholar] [CrossRef]
- Owuor, E.D.; Kon, A.N. Antioxidants and oxidants regulated signal transduction pathways. Biochem. Pharm. 2002, 64, 765–770. [Google Scholar] [CrossRef]
- Chen, Y.C.; Liang, Y.C.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Inhibition of TPA-Induced Protein Kinase C and Transcription Activator Protein-1 Binding Activities by Theaflavin-3,3‘-digallate from Black Tea in NIH3T3 Cells. J. Agric. Food Chem. 1999, 47, 1416–1421. [Google Scholar] [CrossRef]
- Dhandapani, K.M.; Mahesh, V.B.; Brann, D.W. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFκB transcription factors. J. Neurochem. 2007, 102, 522–538. [Google Scholar] [CrossRef]
- Hakimuddin, F.; Tiwari, K.; Paliyath, G.; Meckling, K. Grape and wine phenolic compounds down-regulate the expression of signal transduction genes and inhibit the growth of estrogen receptor—negative MDA-MB231 tumors in nu/nu mouse xenografts. Nutr. Res. 2008, 28, 702–713. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.; Breitkreutz, D.; Hornung, J.; Markham, A. Normal keratinization in a spontaneously immortalized aneuploid keratinocyte cell line. J. Cell. Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1973, 65, 53–63. [Google Scholar]
- Lee, B.L.; Ong, C.N. Comparative analysis of tea catechins and theaflavins by highperformance liquid chromatography and capillary electrophoresis. J. Chromatogr. 2000, 881, 439–447. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds of interest are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kucekova, Z.; Mlcek, J.; Humpolicek, P.; Rop, O.; Valasek, P.; Saha, P. Phenolic Compounds from Allium schoenoprasum, Tragopogon pratensis and Rumex acetosa and Their Antiproliferative Effects. Molecules 2011, 16, 9207-9217. https://doi.org/10.3390/molecules16119207
Kucekova Z, Mlcek J, Humpolicek P, Rop O, Valasek P, Saha P. Phenolic Compounds from Allium schoenoprasum, Tragopogon pratensis and Rumex acetosa and Their Antiproliferative Effects. Molecules. 2011; 16(11):9207-9217. https://doi.org/10.3390/molecules16119207
Chicago/Turabian StyleKucekova, Zdenka, Jiri Mlcek, Petr Humpolicek, Otakar Rop, Pavel Valasek, and Petr Saha. 2011. "Phenolic Compounds from Allium schoenoprasum, Tragopogon pratensis and Rumex acetosa and Their Antiproliferative Effects" Molecules 16, no. 11: 9207-9217. https://doi.org/10.3390/molecules16119207
APA StyleKucekova, Z., Mlcek, J., Humpolicek, P., Rop, O., Valasek, P., & Saha, P. (2011). Phenolic Compounds from Allium schoenoprasum, Tragopogon pratensis and Rumex acetosa and Their Antiproliferative Effects. Molecules, 16(11), 9207-9217. https://doi.org/10.3390/molecules16119207




