Genetic Diversity and Identification of Chinese-Grown Pecan Using ISSR and SSR Markers
Abstract
:1. Introduction
2. Results and Discussion
| No. | Name | Type a | Origin b | Pollination | Harvest Season | Scab Resistance | Nuts/lb | % Kernel | Yield Per Plant (Kg) | Nut Dimension (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Farley | AC | TE | Protogynous | Mid | Excellent | 39 | 47 | 12 | 26 |
| 2 | Schley | AC | TE | Protogynous | Mid | Excellent | 49 | 48 | 9 | 24 |
| 3 | Cape Fear | AC | AG | Protandrous | Early | Good | 55 | 55 | 11 | 27 |
| 4 | Sanber | AC | AG | Protogynous | Mid | Excellent | 37 | 55 | 14 | 20 |
| 5 | Wichita | AC | AG | Protogynous | Mid | Excellent | 45 | 64 | 9 | 21 |
| 6 | Kanza | AC | AG | Protogynous | Mid | Excellent | 49 | 58 | 12 | 20 |
| 7 | Cheyenne | AC | AG | Protandrous | Early | Excellent | 61 | 47 | 16 | 24 |
| 8 | Elliot | AC | AG | Protogynous | Mid-late | Excellent | 71 | 53 | 11 | 22 |
| 9 | Mohawk | AC | AG | Protogynous | Early | Good | 62 | 48 | 16 | 21 |
| 10 | Western | AC | AG | Protogynous | Early | Good | 56 | 44 | 18 | 23 |
| 11 | Mahan | AC | TE | Protogynous | Mid | Good | 51 | 57 | 13 | 25 |
| 12 | Shoshoni | AC | TE | Protogynous | Early | Good | 56 | 54 | 13 | 24 |
| 13 | Starking Hardy Giant | AC | UN | Protandrous | Mid | Good | 61 | 53 | 9 | 22 |
| 14 | Pawnee | AC | UN | Protandrous | Early | Excellent | 54 | 57 | 16 | 24 |
| 15 | Zhongshan 25 | DC | SS | Protogynous | Early | Good | 53 | 51 | 12 | 22 |
| 16 | Zhongshan 39 | DC | NB | Protogynous | Mid | Good | 48 | 53 | 11 | 23 |
| 17 | Zhongshan 40 | DC | NB | Protandrous | Early | Excellent | 49 | 49 | 10 | 21 |
| 18 | Nanjing 148 | DC | NB | Protogynous | Early | Excellent | 57 | 52 | 11 | 24 |
| 19 | Gan2 | DC | NC | Protogynous | Early | Excellent | 66 | 61 | 8 | 25 |
| 20 | Gan3 | DC | NC | Protogynous | Mid | Excellent | 58 | 58 | 9 | 23 |
| 21 | Gan4 | DC | NC | Protogynous | Early | Excellent | 57 | 52 | 6 | 21 |
| 22 | Gan5 | DC | NC | Protandrous | Early | Excellent | 49 | 54 | 9 | 21 |
| 23 | Gan6 | DC | NC | Protogynous | Early | Good | 51 | 57 | 7 | 22 |
| 24 | Gan8 | DC | NC | Protandrous | Mid | Good | 53 | 55 | 6 | 19 |
| 25 | Jinhua 1 | DC | DL | Protogynous | Early | Excellent | 55 | 56 | 9 | 22 |
| 26 | Huangshan 1 | DC | HS | Protogynous | Mid | Excellent | 43 | 51 | 6 | 23 |
| 27 | IW1 | FP | NB | Protandrous | − | Excellent | 61 | 52 | 20 | 22 |
| 28–67 | IW2, IIE1, IIE4, IIE6, IIE7, IIE8, IIE9, IIE17, IIE18, IIE20, IIW1, IIW2, IIW11, IIW13, IIW13Lv, IIW21, IIIN3, IIIN6, IIIN12, IIIN19, IIIN55, IIIE7, IVE15, IVE16, IVE17, IVW28, IVW29, VE5, VE12, VW5, VI4, N704, W21, Shuita 2, Caoping, Lannan1, S401, Changfu, Changfulv, Mang 1 | FP | NB | Protogynous | − | − | − | − | − | − |
| 68–73 | L1, L2, L3, L4, L5, LW1 | FP | SY | Protogynous | − | − | 49 | − | − | − |
| 74 | LE15 | FP | SY | Protandrous | − | Excellent | 36 | 56 | 29 | 22 |
| 75 | IIE32 | FP | NC | Protogynous | − | Excellent | 50 | 52 | 27 | 25 |
| 76 | Carya cathayensis | OH | JD | Good overlap | Late | Good | 34 | 35 | − | − |
| 77 | Juglans nigra | OH | AG | Good overlap | Late | Good | 33 | 41 | − | − |
2.1. ISSR and SSR Amplification
| Primer name | Primer sequence (5′-3′) | Total bands | Ratio of polymorphic bands | Fragment size range (kb) |
|---|---|---|---|---|
| ISSR-5 | (AC)8TG | 10 | 10/10 (100%) | 0.36–1.95 |
| ISSR-22 | (AC)8AA | 7 | 7/7 (100%) | 0.34–1.89 |
| ISSR-26 | (AC)8CC | 9 | 9/9 (100%) | 0.36–1.38 |
| ISSR-35 | (AG)8TA | 12 | 12/12 (100%) | 0.15–1.64 |
| ISSR-45 | (AC)8GC | 11 | 11/11 (100%) | 0.18–1.53 |
| ISSR-56 | (AG)8TT | 7 | 7/7 (100%) | 0.14–1.28 |
| ISSR-61 | (AG)8GT | 10 | 10/10 (100%) | 0.35–1.44 |
| ISSR-62 | (AG)8CA | 9 | 9/9 (100%) | 0.32–1.27 |
| ISSR-64 | (AG)8CG | 10 | 10/10 (100%) | 0.18–1.08 |
| ISSR-65 | (AG)8CC | 9 | 9/9 (100%) | 0.25–1.07 |
| Total | − | 94 | 94/94 (100%) | − |
| Mean | − | 9.4 | 9.4/9.4 (100%) | − |
| Primer name | GenBank accession | Primer sequence(forward primer 5′ to 3′, reverse primer 5′ to 3′) | Total bands | Ratio of polymorphicbands | Fragment size range (kb) | Annealing temp. (°C) |
|---|---|---|---|---|---|---|
| SSR-4 | AY218217 | AAATGGTGAGGAAGTGAAGATATGAA (forward) | 9 | 9/9 (100%) | 0.09–0.33 | 57 |
| GCCCCTTATACAGTTCTACCTCTCTC (reverse) | ||||||
| SSR-7 | AY218220 | AATGAGATGACTACATACACAAGTCGG (forward) | 18 | 18/18 (100%) | 0.09–0.35 | 59 |
| GGGCTCGCATACCTTCATGA (reverse) | ||||||
| SSR-8 | AY218221 | TGAACTCCAAAAGCCTCCTCTC (forward) | 6 | 6/6 (100%) | 0.11–0.23 | 56 |
| GTATTTGTATTTTTTCCTTGAGCTTTCTC (reverse) | ||||||
| SSR-9 | AY218222 | AAAAGTTTTAGGGTTGTTTGCTCTCT (forward) | 5 | 5/5 (100%) | 0.14–0.26 | 56 |
| GTAAAGCCTACAACCTACAACAGTCTATG (reverse) | ||||||
| SSR-15 | AY218228 | TGTAAATGCGTGCTATTGCTGAT (forward) | 10 | 10/10 (100%) | 0.09–0.28 | 54 |
| GAATAGACAAAGAAACGAAACTCATTGA (reverse) | ||||||
| SSR-16 | AY218229 | TCTTCAGAAAAAACCCTTACCTCTCT (forward) | 10 | 10/10 (100%) | 0.06–0.16 | 56 |
| GAAAAATATAAACTCCCATAGTACCCACAT (reverse) | ||||||
| SSR-18 | AY218231 | GGAGTTGTGGAAGCAGTGGA (forward) | 2 | 2/2 (100%) | 0.08–0.09 | 57 |
| GGACCATAAGAGTTTTGACCCTT (reverse) | ||||||
| SSR-19 | AY218232 | CCCCAACTCAATTACAACCTCTTC (forward) | 10 | 10/10 (100%) | 0.05–0.11 | 55 |
| TGTTCATTCTGCACACACACAA (reverse) | ||||||
| Total | − | − | 70 | 70/70 (100%) | − | − |
| Mean | − | − | 7 | 7/7 (100%) | − | − |
2.2. Analysis of the Genetic Diversity of the Pecan Accessions
| Parameter | ISSR | SSR | ||||||
|---|---|---|---|---|---|---|---|---|
| Cluster Ⅰ | Cluster Ⅱ | Cluster Ⅲ | Cluster Ⅳ | Cluster Ⅰ | Cluster Ⅱ | Cluster Ⅲ | Cluster Ⅳ | |
| N | 69 | 79 | 82 | 94 | 59 | 61 | 64 | 76 |
| P (%) | 73.40 | 84.04 | 87.23 | 100 | 77.63 | 80.26 | 84.21 | 100 |
| A | 1.73 ± 0.44 | 1.84 ± 0.37 | 1.87 ± 0.34 | 2.00 ± 0.00 | 1.78 ± 0.42 | 1.80 ± 0.40 | 1.84 ± 0.37 | 2.00 ± 0.00 |
| Ae | 1.36 ± 0.36 | 1.42 ± 0.37 | 1.44 ± 0.36 | 1.46 ± 0.35 | 1.38 ± 0.33 | 1.38 ± 0.33 | 1.41 ± 0.33 | 1.42 ± 0.32 |
| H | 0.22 ± 0.19 | 0.25 ± 0.19 | 0.26 ± 0.18 | 0.27 ± 0.17 | 0.24 ± 0.17 | 0.24 ± 0.17 | 0.25 ± 0.17 | 0.26 ± 0.16 |
| I | 0.34 ± 0.26 | 0.38 ± 0.25 | 0.40 ± 0.25 | 0.42 ± 0.22 | 0.36 ± 0.25 | 0.36 ± 0.24 | 0.39 ± 0.24 | 0.41 ± 0.21 |
| Gst | 0.8286 | 0.9190 | 0.9729 | 0.9742 | 0.8113 | 0.8987 | 0.9678 | 0.9694 |
| Nm | 0.1035 | 0.0441 | 0.0139 | 0.0133 | 0.1163 | 0.0564 | 0.0166 | 0.0158 |
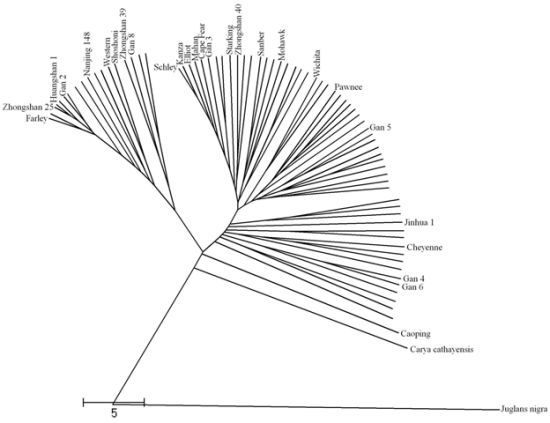
2.3. Dendrogram from ISSR, SSR and Combined Data
| 1 | Farley | - | ||||||||||||||||||||||||
| 2 | Schley | 0.83 | - | |||||||||||||||||||||||
| 3 | Cape Fear | 0.86 | 0.88 | - | ||||||||||||||||||||||
| 4 | Sanber | 0.79 | 0.88 | 0.82 | - | |||||||||||||||||||||
| 5 | Wichita | 0.74 | 0.85 | 0.84 | 0.82 | - | ||||||||||||||||||||
| 6 | Kanza | 0.81 | 0.90 | 0.85 | 0.84 | 0.82 | - | |||||||||||||||||||
| 7 | Cheyenne | 0.66 | 0.83 | 0.72 | 0.76 | 0.79 | 0.83 | - | ||||||||||||||||||
| 8 | Elliot | 0.83 | 0.88 | 0.85 | 0.80 | 0.79 | 0.89 | 0.76 | - | |||||||||||||||||
| 9 | Mohawk | 0.66 | 0.82 | 0.76 | 0.78 | 0.80 | 0.79 | 0.75 | 0.72 | - | ||||||||||||||||
| 10 | Western | 0.84 | 0.84 | 0.85 | 0.78 | 0.76 | 0.80 | 0.69 | 0.86 | 0.71 | - | |||||||||||||||
| 11 | Mahan | 0.81 | 0.86 | 0.83 | 0.79 | 0.79 | 0.90 | 0.78 | 0.88 | 0.75 | 0.82 | - | ||||||||||||||
| 12 | Shoshoni | 0.89 | 0.83 | 0.84 | 0.75 | 0.77 | 0.79 | 0.65 | 0.84 | 0.68 | 0.85 | 0.81 | - | |||||||||||||
| 13 | Starking H G | 0.76 | 0.84 | 0.80 | 0.80 | 0.73 | 0.85 | 0.71 | 0.79 | 0.75 | 0.72 | 0.79 | 0.72 | - | ||||||||||||
| 14 | Pawnee | 0.71 | 0.83 | 0.80 | 0.82 | 0.83 | 0.81 | 0.76 | 0.75 | 0.76 | 0.75 | 0.75 | 0.74 | 0.72 | - | |||||||||||
| 15 | Zhongshan 25 | 0.94 | 0.82 | 0.85 | 0.80 | 0.73 | 0.81 | 0.64 | 0.81 | 0.66 | 0.82 | 0.78 | 0.86 | 0.77 | 0.68 | - | ||||||||||
| 16 | Zhongshan 39 | 0.81 | 0.82 | 0.81 | 0.80 | 0.70 | 0.79 | 0.64 | 0.79 | 0.65 | 0.79 | 0.78 | 0.78 | 0.73 | 0.69 | 0.83 | - | |||||||||
| 17 | Zhongshan 40 | 0.79 | 0.80 | 0.81 | 0.81 | 0.78 | 0.84 | 0.78 | 0.78 | 0.73 | 0.76 | 0.81 | 0.75 | 0.75 | 0.78 | 0.78 | 0.78 | - | ||||||||
| 18 | Nanjing 148 | 0.82 | 0.81 | 0.78 | 0.82 | 0.75 | 0.85 | 0.71 | 0.79 | 0.71 | 0.75 | 0.81 | 0.76 | 0.78 | 0.71 | 0.82 | 0.78 | 0.79 | - | |||||||
| 19 | Gan2 | 0.91 | 0.77 | 0.82 | 0.77 | 0.74 | 0.79 | 0.66 | 0.78 | 0.65 | 0.79 | 0.75 | 0.81 | 0.72 | 0.65 | 0.92 | 0.78 | 0.78 | 0.86 | - | ||||||
| 20 | Gan3 | 0.75 | 0.87 | 0.82 | 0.82 | 0.84 | 0.85 | 0.78 | 0.84 | 0.79 | 0.78 | 0.82 | 0.76 | 0.79 | 0.82 | 0.74 | 0.74 | 0.82 | 0.75 | 0.71 | - | |||||
| 21 | Gan4 | 0.71 | 0.78 | 0.74 | 0.79 | 0.78 | 0.76 | 0.72 | 0.74 | 0.72 | 0.74 | 0.76 | 0.76 | 0.66 | 0.75 | 0.69 | 0.72 | 0.79 | 0.71 | 0.69 | 0.78 | - | ||||
| 22 | Gan5 | 0.64 | 0.79 | 0.76 | 0.76 | 0.78 | 0.79 | 0.78 | 0.72 | 0.79 | 0.68 | 0.73 | 0.66 | 0.73 | 0.81 | 0.63 | 0.68 | 0.78 | 0.68 | 0.59 | 0.84 | 0.71 | - | |||
| 23 | Gan6 | 0.71 | 0.77 | 0.73 | 0.76 | 0.81 | 0.75 | 0.75 | 0.71 | 0.69 | 0.68 | 0.73 | 0.75 | 0.66 | 0.75 | 0.65 | 0.71 | 0.78 | 0.71 | 0.69 | 0.78 | 0.88 | 0.72 | - | ||
| 24 | Gan8 | 0.73 | 0.79 | 0.76 | 0.75 | 0.68 | 0.79 | 0.64 | 0.74 | 0.65 | 0.73 | 0.70 | 0.72 | 0.76 | 0.68 | 0.75 | 0.84 | 0.72 | 0.76 | 0.74 | 0.73 | 0.71 | 0.68 | 0.70 | - | |
| 25 | Jinhua 1 | 0.66 | 0.76 | 0.72 | 0.79 | 0.71 | 0.77 | 0.67 | 0.68 | 0.70 | 0.61 | 0.66 | 0.64 | 0.70 | 0.72 | 0.68 | 0.72 | 0.72 | 0.71 | 0.67 | 0.76 | 0.72 | 0.77 | 0.76 | 0.76 | - |
| 26 | Huangshan 1 | 0.94 | 0.81 | 0.86 | 0.79 | 0.75 | 0.82 | 0.64 | 0.82 | 0.67 | 0.83 | 0.79 | 0.85 | 0.76 | 0.69 | 0.97 | 0.81 | 0.80 | 0.84 | 0.95 | 0.75 | 0.69 | 0.64 | 0.66 | 0.74 | 0.65 |
| Cultivar no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | |

3. Materials and Methods
3.1. Plant Materials
3.2. Total Genomic DNA Extraction and PCR
3.3. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgements
References and notes
- Wetzstein, H.Y.; Rodriguez, A.P.M.; Burns, J.A.; Magner, H.N. Carya illinoensis (Pecan). Biotechnol. Agric. For. 1996, 35, 50–75. [Google Scholar]
- Hou, D.P.; Xi, X.L.; Shi, Z.G. The survey research of Carya illinoensis in China. J. Shandong For. Sci. Technol. 2007, 4, 53–55. [Google Scholar]
- Marquard, R. Isozyme inheritance, polymorphism, and stability of malate dehydrogenase and phosphoglucose isomerase in pecan. J. Am. Soc. Hortic. Sci. 1987, 112, 717–721. [Google Scholar]
- Marquard, R. Rare allozymes of malate dehydrogenase in pecan. HortScience 1989, 24, 156. [Google Scholar]
- Marquard, R. Inheritance of phosphoglucomutase isozymes in pecan. HortScience 1991, 26, 1213–1214. [Google Scholar]
- Marquard, R.; Grauke, L.; Thompson, T.; Janos, R. Identifying pecan cultivars by isozymes and inheritance of leucine aminopeptidase. J. Am. Soc. Hortic. Sci. 1995, 120, 661–666. [Google Scholar]
- Ruter, B.; Hamrick, J.; Wood, B. Genetic diversity within provenance and cultivar germplasm collections and wild populations of pecan. J. Hered. 2000, 90, 521–528. [Google Scholar]
- Ruter, B.; Hamrick, J.; Wood, B. Outcrossing rates and relatedness estimates in pecan populations. J. Hered. 2001, 91, 72–75. [Google Scholar]
- Wood, B.W.; Grauke, L.J.; Payne, J.A. Provenance variation in pecan. J. Am. Soc. Hortic. Sci. 1998, 123, 1023–1028. [Google Scholar]
- Vendrame, W.; Kochert, G.; Sparks, D.; Wetzstein, H. Field performance and molecular evaluations of pecan trees regenerated from somatic embryogenic cultures. J. Am. Soc. Hortic. Sci. 2000, 125, 542–546. [Google Scholar]
- Conner, P.; Wood, B. Identification of pecan cultivars and their genetic relatedness as determined by randomly amplified polymorphic DNA analysis. J. Am. Soc. Hortic. Sci. 2001, 126, 474–480. [Google Scholar]
- Grauke, L.; Iqbal, M.; Reddy, A.; Thompson, T. Developing microsatellite DNA markers in pecan. J. Am. Soc. Hortic. Sci. 2003, 128, 374–380. [Google Scholar]
- Beedanagari, S.R.; Dove, S.K.; Wood, B.W.; Conner, P.J. A first linkage map of pecan cultivars based on RAPD and AFLP markers. Theor. Appl. Genet. 2005, 110, 1127–1137. [Google Scholar] [CrossRef]
- Zhang, R.Q.; He, F.; Lv, F.D.; Li, B. Population genetic analysis by randomly amplified polymorphic DNA markers in pecan. Econ. For. Res. 2001, 19, 1–6. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Lv, F.D.; Tan, X.F.; He, F.; Xie, B.X. Identification of main varieties of American hickory by RAPD. Nonwood For. Res. 2004, 22, 1–5. [Google Scholar]
- Djè, Y.; Tahi, C.G.; Zoro, Bi, A.I.; Baudoin, J.-P.; Bertin, P. Use of ISSR markers to assess genetic diversity of African edible seeded Citrullus lanatus landraces. Sci. Hortic. 2010, 124, 159–164. [Google Scholar]
- Nas, M.N.; Bolek, Y.; Bardak, A. Genetic diversity and phylogenetic relationships of Prunus microcarpa C.A. Mey. subsp. tortusa analyzed by simple sequence repeats (SSRs). Sci. Hortic. 2011, 127, 220–227. [Google Scholar] [CrossRef]
- Pomper, K.W.; Lowe, J.D.; Lu, L.; Crabtree, S.B.; Dutta, S. Characterization and identification of Pawpaw cultivars and advanced selections by simple sequence repeat markers. J. Am. Soc. Hortic. Sci. 2010, 135, 143–149. [Google Scholar]
- Castro, I.; Martin, J.P.; Ortiz, J.M.; Pinto-Carnide, O. Varietal discrimination and genetic relationships of Vitis vinifera L. cultivars from two major Controlled Appellation (DOC) regions in Portugal. Sci. Hortic. 2011, 127, 507–514. [Google Scholar] [CrossRef]
- Williams, J.G.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990, 18, 6531–6535. [Google Scholar]
- Wünsch, A.; Hormaza, J.J. Cultivar identification and genetic fingerprinting of temperate fruit tree species using DNA markers. Euphytica 2002, 125, 59–67. [Google Scholar] [CrossRef]
- Wang, A.H.; Yu, Z.Y.; Ding, Y. Genetic diversity analysis of wild close relatives of barley from Tibet and the Middle East by ISSR and SSR markers. C. R. Biol. 2009, 332, 393–403. [Google Scholar] [CrossRef]
- Tantasawat, P.; Trongchuen, J.; Prajongjai, T.; Seehalak, W.; Jittayasothorn, Y. Variety identification and comparative analysis of genetic diversity in yardlong bean (Vigna unguiculata spp. sesquipedalis) using morphological characters, SSR and ISSR analysis. Sci. Hortic. 2010, 124, 204–216. [Google Scholar]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef]
- Siragusa, M.; Pasquale, F.; Abbate, L.; Tusa, N. Identification of sour orange accessions and evaluation of their genetic variability by molecular marker analyses. HortScience 2006, 41, 84–89. [Google Scholar]
- KenKnight, G.E. Pecan varieties “happen” in Jackson County, Mississippi. Pecan Q. 1970, 4, 6–7. [Google Scholar]
- Zhao, K.G.; Zhou, M.Q.; Chen, L.Q. Genetic diversity and discrimination of Chimonanthus praecox (L.) link germplasm using ISSR and RAPD markers. HortScience 2007, 42, 1144–1148. [Google Scholar]
- Bassam, B.J.; Caetano-Anolles, G. Sliver staining of DNA in polyacrylamide gels. Appl. Biochem. Biotechnol. 1993, 42, 181–188. [Google Scholar] [CrossRef]
- Yeh, F.C.; Yang, R.C.; Boyle, T. Popgene version 1.31. Microsoft Windows based freeware for population genetic analysis. University of Alberta and Tim Boyle, Centre for International Forestry Research, Edmonton, Alta, Canada, 1999.
- Sample Availability: Samples of the 77 accessions are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jia, X.-D.; Wang, T.; Zhai, M.; Li, Y.-R.; Guo, Z.-R. Genetic Diversity and Identification of Chinese-Grown Pecan Using ISSR and SSR Markers. Molecules 2011, 16, 10078-10092. https://doi.org/10.3390/molecules161210078
Jia X-D, Wang T, Zhai M, Li Y-R, Guo Z-R. Genetic Diversity and Identification of Chinese-Grown Pecan Using ISSR and SSR Markers. Molecules. 2011; 16(12):10078-10092. https://doi.org/10.3390/molecules161210078
Chicago/Turabian StyleJia, Xiao-Dong, Tao Wang, Min Zhai, Yong-Rong Li, and Zhong-Ren Guo. 2011. "Genetic Diversity and Identification of Chinese-Grown Pecan Using ISSR and SSR Markers" Molecules 16, no. 12: 10078-10092. https://doi.org/10.3390/molecules161210078