Microwave-Assisted Extraction of Phenolic Antioxidants from Potato Peels
Abstract
:Abbreviations
| Phentot | total phenolic content |
| AA | ascorbic acid |
| ChloA | chlorogenic acid |
| CafA | caffeic acid |
| FerA | ferulic acid |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| MAE | microwave-assisted extraction |
| RSM | response surface methodology |
| R2 | Correlation coefficient |
1. Introduction
2. Results and Discussion
2.1. Optimization of conditions for phenolic extraction
| Source | SS | DF | MS | F-Value | p-value |
|---|---|---|---|---|---|
| Model | 2.83 | 4 | 0.71 | 56.34 | ≤0.0001 |
| S-Solvent Concentration | 0.45 | 1 | 0.45 | 35.90 | ≤0.0001 |
| t-Time | 0.18 | 1 | 0.18 | 14.37 | 0.0026 |
| MP- Power | 1.009 × 10−3 | 1 | 1.009 × 10−3 | 0.21 | 0.6636 |
| S×t | 5.065 × 10−3 | 1 | 5.065 × 10−3 | 1.04 | 0.3428 |
| S×MP | 3.176 × 10−7 | 1 | 3.176 × 10−7 | 6.942 × 10−5 | 0.9938 |
| T×MP | 1.788 × 10−4 | 1 | 1.788 × 10−4 | 0.037 | 0.8538 |
| S2 | 2 | 1 | 2 | 159.64 | ≤0.0001 |
| t2 | 0.078 | 1 | 0.078 | 6.20 | 0.0284 |
| P2 | 0.020 | 1 | 0.020 | 4.11 | 0.0821 |
| Residual | 0.15 | 12 | 0.013 | ||
| Lack of Fit | 0.13 | 8 | 0.016 | 2.69 | 0.1773 |
| R2 | 0.95 |







2.2. Radical scavenging activity

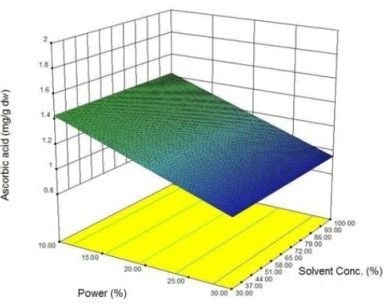
2.3. Response surface analysis

| Component | Present study | Reported literature | Reference and study group |
|---|---|---|---|
| Quantified | |||
| Phentot | 3.94 ± 0.21 mg /g dw | 1.51 ± 0.17 − 3.31 ± 0.12 mg/g dw | [11] |
| AA | 1.44 ± 0.5 mg /g dw | - | - |
| ChloA | 1.35 ± 0.18 mg /g dw | 0.78 ± 0.01 − 2.79 ± 0.12 mg/g dw | [11] |
| CafA | 1.33 ± 0.06 mg /g dw | 0.26 ± 0.01 − 0.72 ± 0.29 mg/g dw | [11] |
| FerA | 0.5 ± 0.02 mg /g dw | 0.6 ± 0.1 – 3.9 ± 0.4 mg/100 g dw | [29] |
| DPPH | 74 ± 5.5% | - | - |

| Predicted responses | Methanol concentration (%v/v) | Extraction time (min) | Power level (W) | Predicted content mg g−1/ dw |
|---|---|---|---|---|
| AA | 100 | 15 | 10 | 1.44 ± 0.5 |
| ChloA | 100 | 5 | 10 | 1.35 ± 0.18 |
| CafA | 100 | 15 | 10 | 1.33 ± 0.06 |
| FerA | 100 | 15 | 10 | 0.5 ± 0.02 |
| Phentot | 67.33 | 15 | 14.67 | 3.94 ± 0.21 |
| DPPH | 100 | 5 | 10 | 74 ± 5.5% |
3. Experimental
3.1. Materials and reagents
3.2. Equipment and apparatus

3.3. Preparation of potato peel extracts
3.4. Determination of total phenolic compounds
3.5. Scavenging activity on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals

3.6. HPLC analysis


3.7. Optimization of conditions for microwave-assisted extraction of phenolics
| Code | Solvent concentration (%v/v) | Time (min) | Power (%) |
|---|---|---|---|
| -1 | 30 | 5 | 10 |
| 0 | 65 | 10 | 20 |
| 1 | 100 | 15 | 30 |
3.8. Statistical analysis
| Run | Methanol concentration (%v/v) | Time (min) | Power (%) | Total Phenolic (mg/g dw) | Ascorbic acid content (mg/g dw) | Chlorogenic acid content (mg/g dw) | Caffeic acid content (mg/g dw) | Ferulic acid content (mg/g dw) | DPPH (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 3.626 | 1.109 | 0.62 | 0.63 | 0.34 | 57.27 |
| 2 | 0 | 0 | -1 | 3.504 | 1.125 | 0.465 | 0.581 | 0.37 | 55.20 |
| 3 | 0 | 0 | 0 | 3.733 | 0.917 | 0.571 | 0.627 | 0.288 | 56.65 |
| 4 | 0 | 0 | 0 | 3.605 | 1.062 | 0.725 | 0.961 | 0.367 | 55.5 |
| 5 | -1 | 0 | 0 | 2.595 | 1.025 | 0.258 | 0.284 | 0.162 | 34.03 |
| 6 | 0 | 0 | 0 | 3.701 | 1.492 | 0.658 | 0.742 | 0.319 | 57.55 |
| 7 | -1 | 1 | -1 | 2.964 | 1.577 | 0.362 | 0.642 | 0.238 | 42.35 |
| 8 | 0 | -1 | 0 | 3.729 | 1.087 | 0.532 | 0.59 | 0.23 | 68.55 |
| 9 | 1 | -1 | -1 | 3.151 | 1.478 | 1.674 | 1.524 | 0.31 | 70.66 |
| 10 | -1 | -1 | -1 | 2.051 | 0.92 | 0.328 | 0.228 | 0.16 | 52.33 |
| 11 | 1 | 1 | 1 | 3.001 | 1.012 | 0.774 | 0.892 | 0.374 | 70.46 |
| 12 | 0 | 0 | 0 | 3.515 | 1.097 | 0.435 | 0.493 | 0.342 | 56.67 |
| 13 | 1 | 1 | -1 | 3.21 | 1.915 | 1.524 | 1.411 | 0.381 | 73.32 |
| 14 | 0 | 1 | 0 | 3.924 | 1.167 | 0.861 | 0.92 | 0.44 | 72.94 |
| 15 | -1 | -1 | 1 | 2.37 | 0.917 | 0.37 | 0.288 | 0.16 | 41.4 |
| 16 | -1 | 1 | 1 | 2.969 | 1.02 | 0.279 | 0.407 | 0.206 | 48.66 |
| 17 | 1 | 0 | 0 | 3.12 | 1.106 | 1.033 | 1.19 | 0.396 | 66.45 |
| 18 | 1 | -1 | 1 | 3.067 | 1.262 | 1.392 | 1.228 | 0.419 | 69.5 |
| 19 | 0 | 0 | 0 | 3.675 | 1.51 | 0.521 | 0.491 | 0.427 | 72.34 |
| 20 | 0 | 0 | 1 | 3.541 | 0.954 | 0.585 | 0.57 | 0.317 | 86.23 |

4. Conclusions
Acknowledgements
References and Notes
- Nelson, M.L. Utilization and application of wet potato processing coproducts for finishing cattle. J. Anim. Sci. 2010, 88, E133–E142. [Google Scholar] [CrossRef]
- Zia-ur, R.; Habib, F.; Shah, W.H. Utilization of potato peels extract as a natural antioxidant in soy bean oil. Food Chem. 2004, 85, 215–220. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Radhakrishna, P.; Sharma, A. Potato peel extract - A natural antioxidant for retarding lipid peroxidation in radiation processed lamb meat. J. Agric. Food Chem. 2005, 53, 1499–1504. [Google Scholar]
- Al-Saikhan, M.S.; Howard, L.R.; Miller, J.C., Jr. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum L.). J. Food Sci. 1995, 60, 341–343. [Google Scholar] [CrossRef]
- Kolasa, K.M. The potato and human nutrition. Am. Potato J. 1993, 70, 375–384. [Google Scholar]
- Lachman, J.; Hamouz, K.; Orsak, M.; Pivec, V.; Dvorak, P. Differences in phenolic content and antioxidant activity in yellow and purple-fleshed potatoes grown in the Czech Republic. Plant Soil Environ. 2008, 54, 1–6. [Google Scholar]
- Lachman, J.; Hamouz, K.; Orsak, M.; Pivec, V.; Dvorak, P. The influence of flesh colour and growing locality on polyphenolic content and antioxidant activity in potatoes. Sci. Hort. 2008, 117, 109–114. [Google Scholar] [CrossRef]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef]
- Singh, N.; Kamath, V.; Rajini, P.S. Protective effect of potato peel powder in ameliorating oxidative stress in streptozotocin diabetic rats. Plant Food. Hum. Nutr. 2005, 60, 49–54. [Google Scholar]
- Lachman, J.; Hamouz, K. Red and purple coloured potatoes as a significant antioxidant source in human nutrition - A review. Plant Soil Environ. 2005, 51, 477–482. [Google Scholar]
- Al-Weshahy, A.; Venket Rao, A. Isolation and characterization of functional components from peel samples of six potatoes varieties growing in Ontario. Food Res. Int. 2009, 42, 1062–1066. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Biochemistry, and Dietary Role of Potato Polyphenol. A Review. J. Agric. Food Chem. 1997, 45, 1523–1540. [Google Scholar] [CrossRef]
- Li, H.B.; Jiang, Y.; Wong, C.C.; Cheng, K.W.; Chen, F. Evaluation of two methods for the extraction of antioxidants from medicinal plants. Anal. Bioanal. Chem. 2007, 388, 483–488. [Google Scholar]
- Safer, A.M. Hepatotoxicity induced by the anti-oxidant food additive, butylated hydroxytoluene (BHT), in rats: An electron microscopical study. Histol. Histopathol. 1999, 14, 391–406. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Rodriguez De Sotillo, D.; Hadley, M.; Holm, E.T. Phenolics in aqueous potato peel extract: Extraction, identification and degradation. J. Food Sci. 1994, 59, 649–651. [Google Scholar]
- Dai, J.; Yaylayan, V.A.; Raghavan, G.S.V.; Paré, J.R. Extraction and colorimetric determination of azadirachtin-related limonoids in neem seed kernel. J. Agric. Food Chem. 1999, 47, 3738–3742. [Google Scholar] [CrossRef]
- Dai, J.; Yaylayan, V.A.; Raghavan, G.S.V.; Paré, J.R.J.; Liu, Z.; Bélanger, J.M.R. Influence of operating parameters on the use of the Microwave-Assisted Process (MAP) for the extraction of azadirachtin-related limonoids from neem (Azadirachta indica) under atmospheric pressure conditions. J. Agric. Food Chem. 2001, 49, 4584–4588. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Wang, Y.L.; Xi, G.S.; Zheng, Y.C.; Miao, F.S. Microwave-assisted extraction of flavonoids from Chinese herb Radix puerariae (Ge Gen). J. Med. Plant Res. 2010, 4, 304–308. [Google Scholar]
- Rubilar, M.; Pinelo, M.; Franco, D.; Sineiro, J.; Nunez, M.J. Agroindustrial residues as a source of antioxidants-Residuos agroindustriales como fuente de antioxidantes. Afinidad 2003, 60, 153–160. [Google Scholar]
- Nemes, S.M.; Orsat, V. Microwave-Assisted Extraction of Secoisolariciresinol Diglucoside-Method Development. Food Bioprocess Technol. 2009, 1–9. [Google Scholar]
- Nemes, S.; Orsat, V. Screening the Experimental Domain for the Microwave-Assisted Extraction of Secoisolariciresinol Diglucoside from Flaxseed Prior to Optimization Procedures. Food Bioprocess Technol. 2010, 3, 300–307. [Google Scholar] [CrossRef]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S.F. Optimizing the extraction of phenolic antioxidants from peanut skins using response surface methodology. J. Agric. Food Chem. 2009, 57, 3064–3072. [Google Scholar]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O'Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Chen, T.; Sun, X.; Xiao, W.; Liu, X.; Zhang, W.; Ma, K.; Zhu, Y. Optimization of microwave-assisted extraction of solanesol from potato leaves and stems. Med. Chem. Res. 2010, 19, 732–742. [Google Scholar] [CrossRef]
- Csiktusnádi Kiss, G.A.; Forgacs, E.; Cserhati, T.; Mota, T.; Morais, H.; Ramos, A. Optimisation of the microwave-assisted extraction of pigments from paprika (Capsicum annuum L.) powders. J. Chromatogr. A 2000, 889, 41–49. [Google Scholar] [CrossRef]
- Stushnoff, C.; Holm, D.; Thompson, M.; Jiang, W.; Thompson, H.; Joyce, N.; Wilson, P. Antioxidant Properties of Cultivars and Selections from the Colorado Potato Breeding Program. Am. J. Potato Res. 2008, 85, 267–276. [Google Scholar] [CrossRef]
- Leo, L.; Leone, A.; Longo, C.; Lombardi, D.A.; Raimo, F.; Zacheo, G. Antioxidant compounds and antioxidant activity in "early potatoes". J. Agric. Food Chem. 2008, 56, 4154–4163. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, B.; Chen, Y.; Xie, M.; Duan, H.; Li, Y.; Duan, G. Nitrogen-protected microwave-assisted extraction of ascorbic acid from fruit and vegetables. J. Sep. Sci. 2009, 32, 4227–4233. [Google Scholar]
- Augustin, J.; Mcdole, R.E.; Mcmaster, G.M.; Painter, C.G.; Sparks, W.C. Ascorbic acid content in Russet Burbank potatoes. J. Food Sci. 1975, 40, 415–416. [Google Scholar] [CrossRef]
- Burgos, G.; Auqui, S.; Amoros, W.; Salas, E.; Bonierbale, M. Ascorbic acid concentration of native Andean potato varieties as affected by environment, cooking and storage. J. Food Compos. Anal. 2009, 22, 533–538. [Google Scholar] [CrossRef]
- Hyon, W.I.; Suh, B.S.; Lee, S.U.; Kozukue, N.; Ohnisi-Kameyama, M.; Levin, C.E.; Friedman, M. Analysis of phenolic compounds by high-performance liquid chromatography and liquid chromatography/mass spectrometry in potato plant flowers, leaves, stems, and tubers and in home-processed potatoes. J. Agric. Food Chem. 2008, 56, 3341–3349. [Google Scholar]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; De Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.P. Determination of DPPH radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef]
- Liao, X.; Raghavan, G.S.V.; Yaylayan, V.A. Dielectric properties of alcohols (C1-C5) at 2450 MHz and 915 MHz. J. Mol. Liquids 2001, 94, 51–60. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Tomasik, P. Viscosity and dielectric properties of liquid binary mixtures. J. Phys. Org. Chem. 1990, 3, 493–502. [Google Scholar]
- Venkatesh, M.S.; Raghavan, G.S.V. An Overview of Microwave Processing and Dielectric Properties of Agri-food Materials. Biosyst. Eng. 2004, 88, 1–18. [Google Scholar] [CrossRef]
- Cheng, W.M.; Raghavan, G.S.V.; Ngadi, M.; Wang, N. Microwave power control strategies on the drying process I. Development and evaluation of new microwave drying system. J. Food Eng. 2006, 76, 188–194. [Google Scholar] [CrossRef]
- Jagadeesh, S.L.; Charles, M.T.; Gariepy, Y.; Goyette, B.; Raghavan, G.S.V.; Vigneault, C. Influence of postharvest UV-C hormesis on the bioactive components of tomato during post-treatment handling. Food Bioprocess Technol. 2009, 1–10. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977, 28, 49–55. [Google Scholar]
- Nair, V.D.P.; Dairam, A.; Agbonon, A.; Arnason, J.T.; Foster, B.C.; Kanfer, I. Investigation of the antioxidant activity of African potato (Hypoxis hemerocallidea). J. Agric. Food Chem. 2007, 55, 1707–1711. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the experimental attainment of optimum conditions. J. Roy. Stat. Soc. 1951, 13, 1–45. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Singh, A.; Sabally, K.; Kubow, S.; Donnelly, D.J.; Gariepy, Y.; Orsat, V.; Raghavan, G.S.V. Microwave-Assisted Extraction of Phenolic Antioxidants from Potato Peels. Molecules 2011, 16, 2218-2232. https://doi.org/10.3390/molecules16032218
Singh A, Sabally K, Kubow S, Donnelly DJ, Gariepy Y, Orsat V, Raghavan GSV. Microwave-Assisted Extraction of Phenolic Antioxidants from Potato Peels. Molecules. 2011; 16(3):2218-2232. https://doi.org/10.3390/molecules16032218
Chicago/Turabian StyleSingh, Ashutosh, Kebba Sabally, Stan Kubow, Danielle J. Donnelly, Yvan Gariepy, Valérie Orsat, and G.S.V. Raghavan. 2011. "Microwave-Assisted Extraction of Phenolic Antioxidants from Potato Peels" Molecules 16, no. 3: 2218-2232. https://doi.org/10.3390/molecules16032218