Bismuth(III) Reagents in Steroid and Terpene Chemistry
Abstract
:1. Introduction
2. Oxidation Reactions
2.1. Oxidation of α-hydroxyketones by Bi2O3




2.2. Allylic oxidation


2.3. Oxidation of alcohols


3. Formation of Common Protecting Groups
3.1. Acylation of alcohols
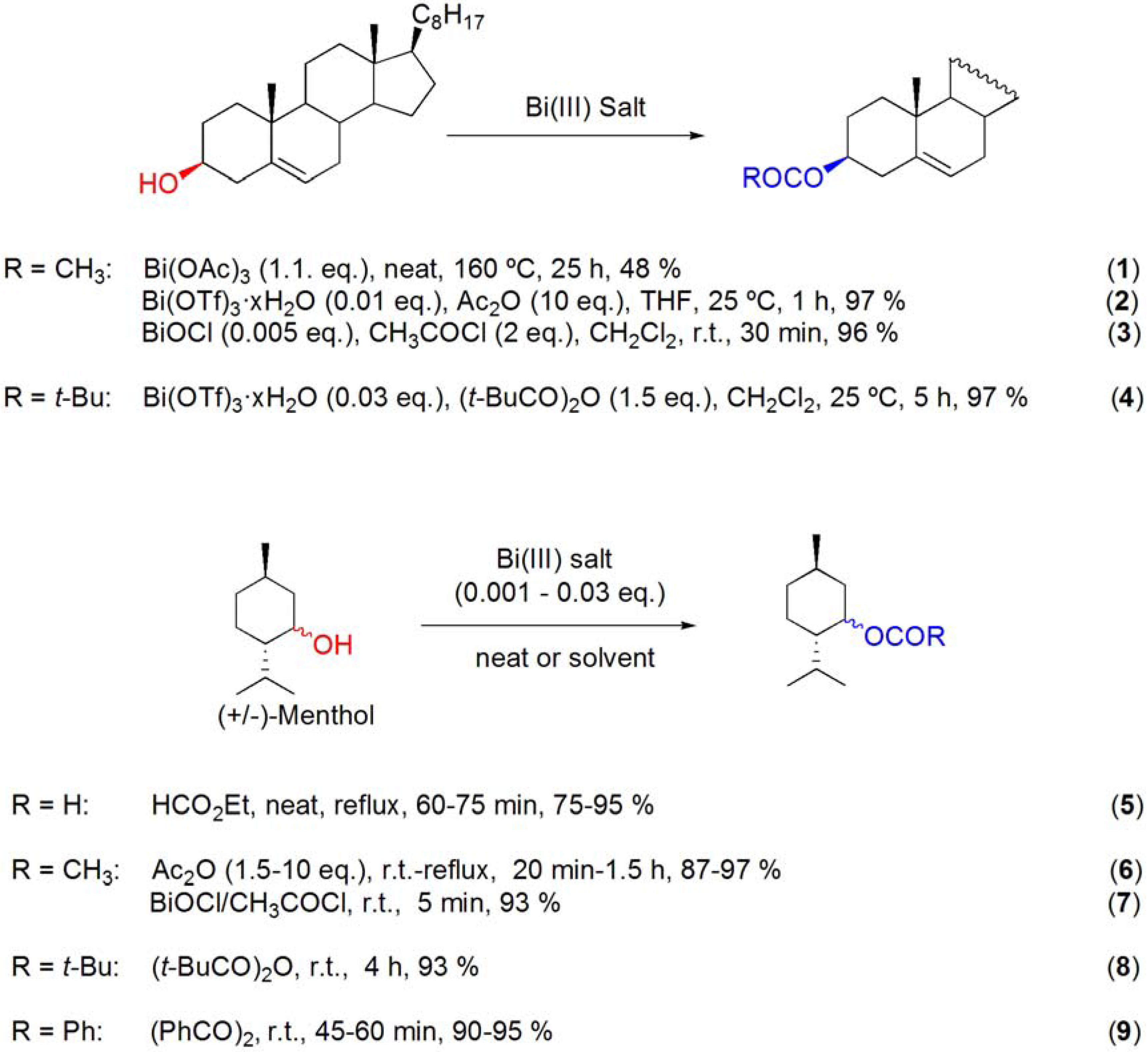
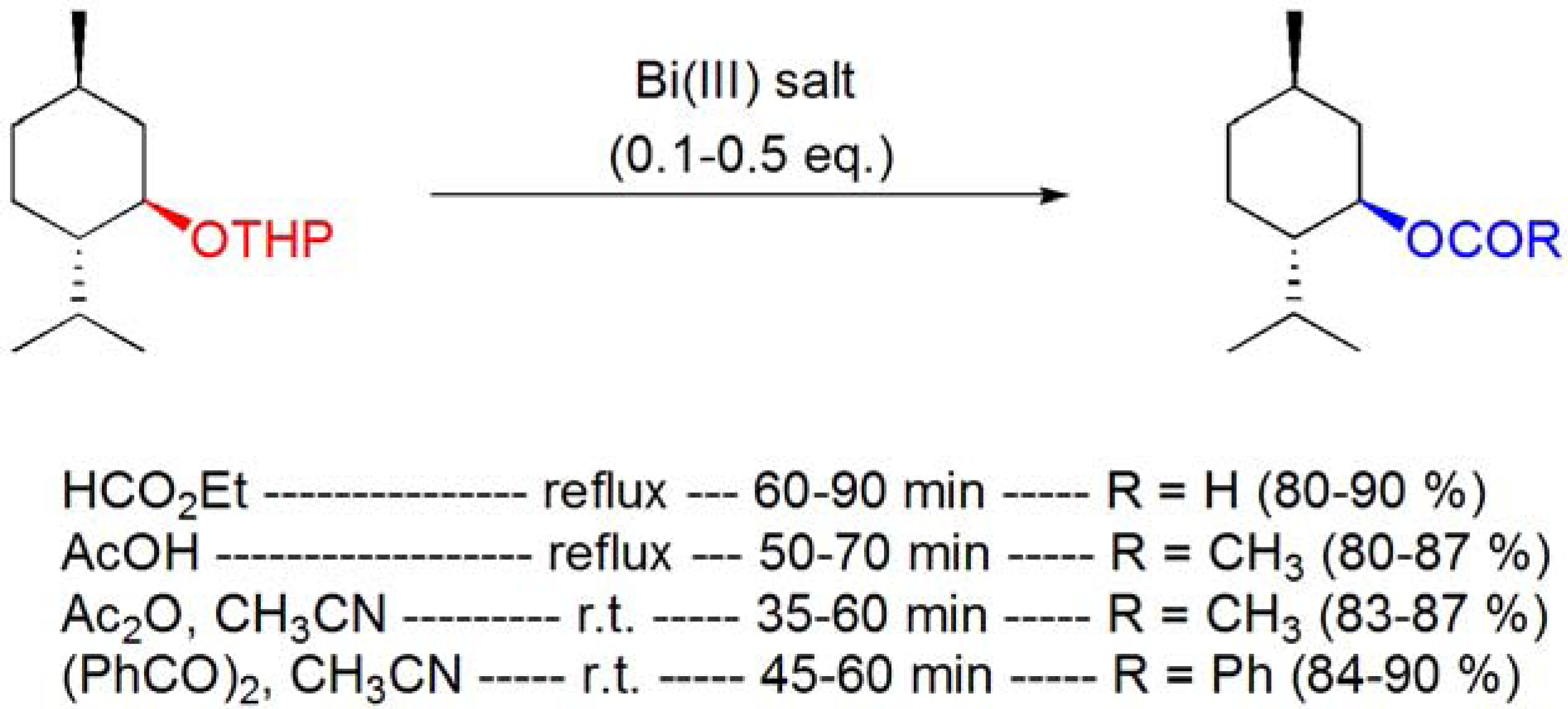

3.2. Formation of ethers


4. Removal of Common Protecting Groups
4.1. Deprotection of O,O-acetals

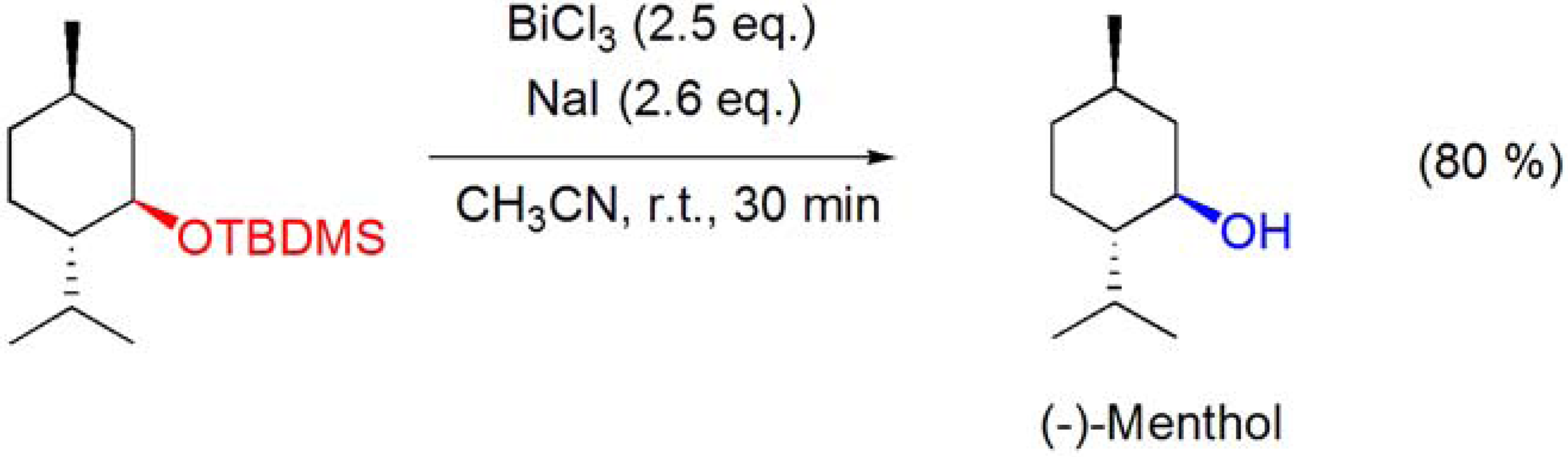
4.2. Deprotection of ether groups

4.3. Deprotection of oximes to carbonyl compounds

5. Nucleophilic Ring-opening of Epoxides
5.1. Ritter reaction of epoxides


5.2. Ring opening of epoxides with bismuth(III) salt
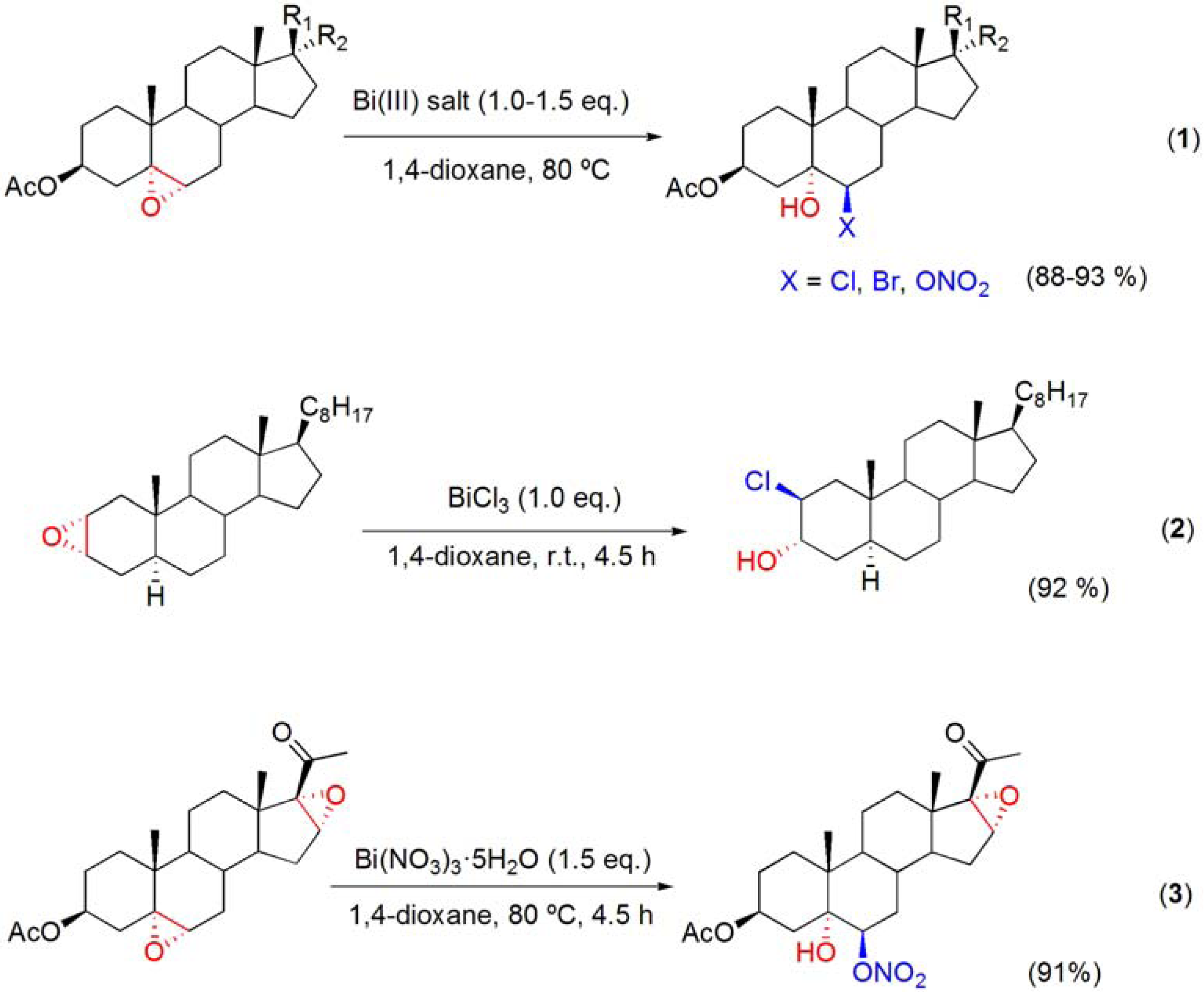

5.3. Trans-diaxial hydroxylation of Δ5-steroids

6. Rearrangement Reactions
6.1. Ferrier rearrangement

6.2. Epoxyolefin cyclizations

6.3. Westphalen and “Backbone” rearrangements
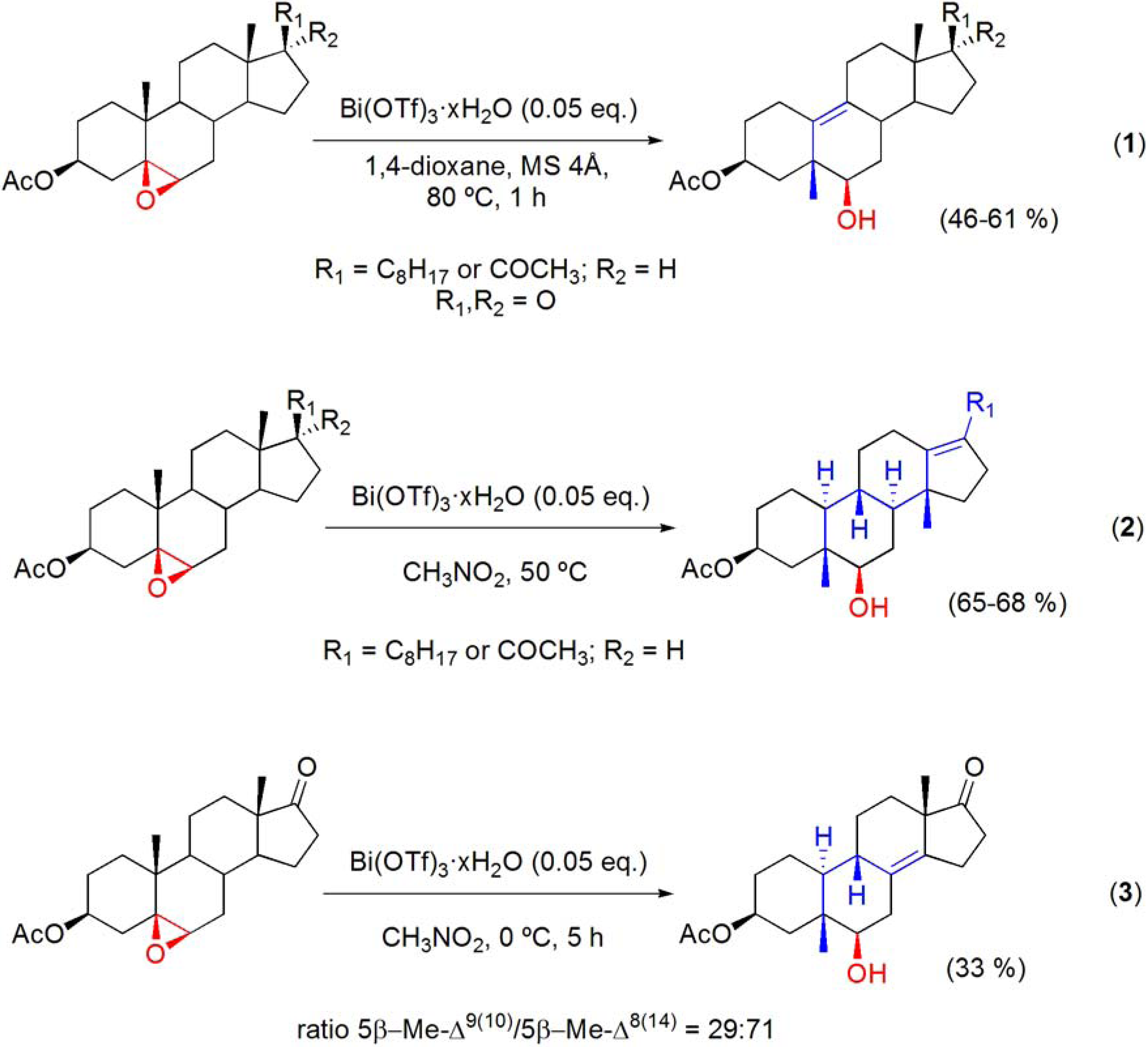

6.4. Wagner-Meerwein rearrangements







6.5. Oxidative rearrangement of tertiary allylic alcohols

7. Miscellaneous Reactions
7.1. Diels-Alder cycloaddition

7.2. Ene reaction


7.3. Aromatic nitration reactions using Bi(NO3)3·5H2O

7.4. Lactonization of Δ12-oleananes

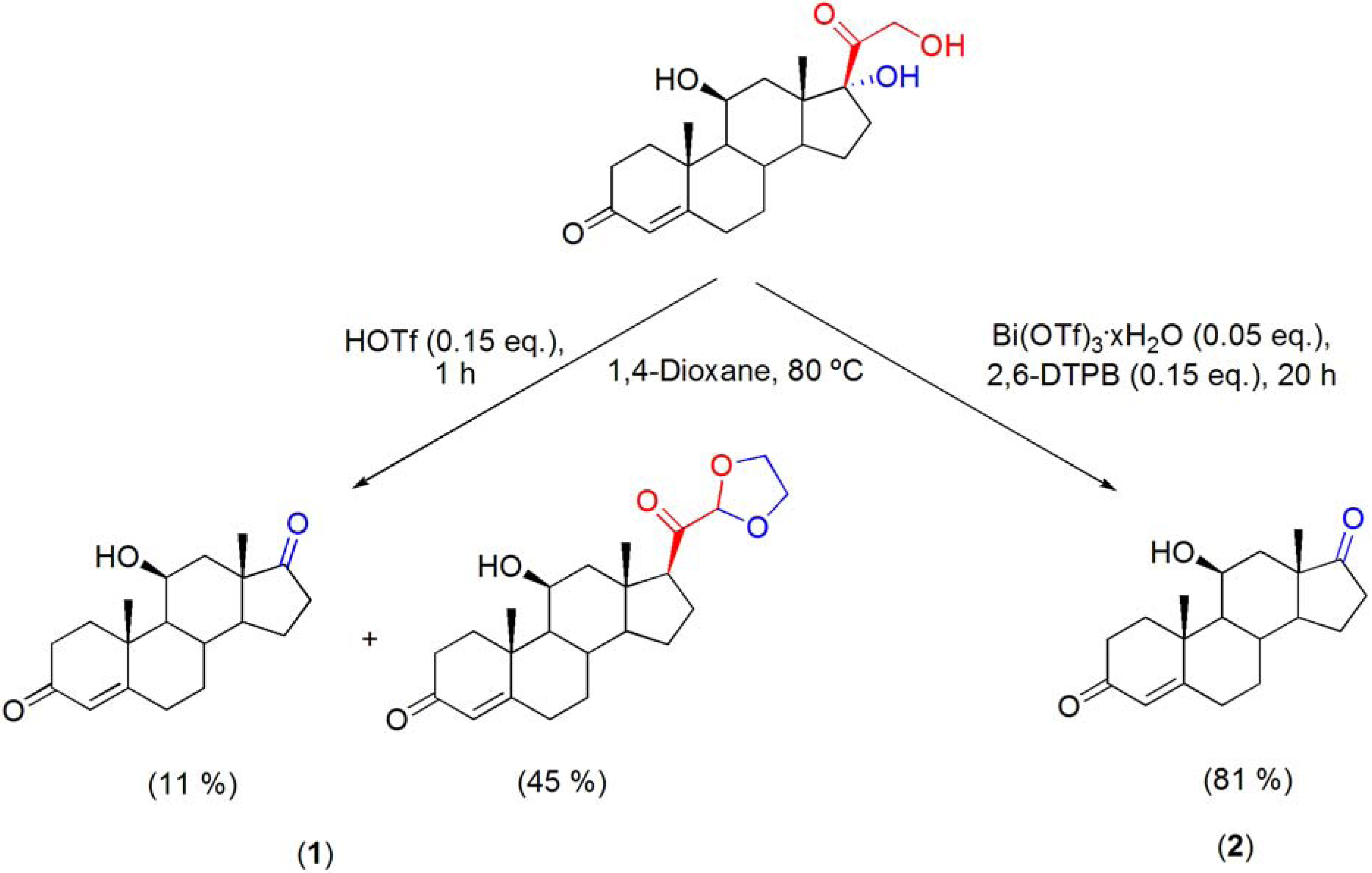
7.5. Cleavage of the C17-side chain of corticosteroids

4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
References and Notes
- Hanson, J.R. Steroids: Partial synthesis in medicinal chemistry. Nat. Prod. Rep. 2010, 27, 887–899. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural product in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Trost, B.M. Atom economy - A challenge for organic synthesis: Homogeneous catalysis leads the way. Angew. Chem. Int. Ed. 1995, 34, 259–281. [Google Scholar] [CrossRef]
- Clark, J.H. Catalysis for green chemistry. Pure Appl. Chem. 2001, 73, 103–111. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Horvath, I.T.; Anastas, P.T. Innovations and green chemistry. Chem. Rev. 2007, 107, 2169–2173. [Google Scholar] [CrossRef]
- Sheldon, R.A. E factors, green chemistry and catalysis: An odyssey. Chem. Commun. 2008, 3352–3365. [Google Scholar] [CrossRef]
- Postel, M.; Duñach, E. Bismuth derivatives for the oxidation of organic compounds. Coord. Chem. Rev. 1996, 155, 127–144. [Google Scholar] [CrossRef]
- Marshall, J.A. Organic chemistry of Bi(III) compounds. Chemtracts: Org. Chem. 1997, 1064–1075. [Google Scholar]
- Suzuki, H.; Ikegami, T.; Matano, Y. Bismuth in organic transformations. Synthesis 1997, 249–267. [Google Scholar]
- Komatsu, N. Bismuth compounds in organic transformations. In Organobismuth Chemistry; Suzuki, H., Matano, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 371–440. [Google Scholar]
- Vidal, S. Bismuth(III) derivatives: New catalysts. Synlett 2001, 1194–1195. [Google Scholar] [CrossRef]
- Le Roux, C.; Dubac, J. Bismuth(III) chloride and triflate: Novel catalysts for acylation and sulfonylation reactions. Survey and mechanistic aspects. Synlett 2002, 181–200. [Google Scholar] [CrossRef]
- Leonard, N.M.; Wieland, L.C.; Mohan, R.S. Applications of bismuth(III) compounds in organic synthesis. Tetrahedron 2002, 58, 8373–8397. [Google Scholar] [CrossRef]
- Antoniotti, S. Bismuth(III) tris(trifluoromethanesulfonate). Synlett 2003, 1566–1567. [Google Scholar] [CrossRef]
- Antoniotti, S.; Duñach, E. Recent uses of bismuth derivatives in organic synthesis. C. R. Chim. 2004, 7, 679–688. [Google Scholar] [CrossRef]
- Gaspard-Iloughmane, H.; Le Roux, C. Bismuth(III) triflate in organic synthesis. Eur. J. Org. Chem. 2004, 2517–2532. [Google Scholar] [CrossRef]
- Loh, T.-P.; Chua, G.-L. Activation of reactions by Lewis acids derived from Ga, In, Sb and Bi. In Advances in Organic Synthesis; Atta-ur-Rahman, Ed.; Bentham Science Publishers: Oak Park, IL, USA, 2005; Volume 1, pp. 173–214. [Google Scholar]
- Mulamoottil, V.A. Bismuth(III) nitrate pentahydrate—A versatile reagent in organic synthesis. Synlett 2005, 2699–2700. [Google Scholar] [CrossRef]
- Gaspard-Iloughmane, H.; Le Roux, C. Recent advances in the use of bismuth(III) triflate in organic synthesis: an update. Trends Org. Chem. 2006, 11, 65–80. [Google Scholar]
- Gaspard-lloughmane, H.; Le Roux, C. Bismuth(III) lewis acids. In Acid Catalysis for Modern Organic Synthesis; Yamamoto, H., Ishihara, K., Eds.; Wiley: New York, NY, USA, 2008; pp. 551–588. [Google Scholar]
- Hua, R.M. Recent advances in bismuth-catalyzed organic synthesis. Curr. Org. Synth. 2008, 5, 1–27. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Pinto, R.M.A.; Silvestre, S.M. Recent Advances of Bismuth(III) Salts in Organic Chemistry: Application to the Synthesis of Aliphatics, Alicyclics, Aromatics, Amino Acids and Peptides, Terpenes and Steroids of Pharmaceutical Interest. Mini-Rev. Org. Chem. 2009, 6, 241–274. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Pinto, R.M.A.; Silvestre, S.M. Recent Advances of Bismuth(III) Salts in Organic Chemistry: Application to the Synthesis of Heterocycles of Pharmaceutical Interest. Curr. Org. Synth. 2009, 6, 426–470. [Google Scholar] [CrossRef]
- Yamamoto, H.; Futatsugi, K. "Designer acids": Combined acid catalysis for asymmetric synthesis. Angew. Chem. Int. Ed. 2005, 44, 1924–1942. [Google Scholar] [CrossRef]
- Holden, B.; Rigby, W. The bismuth-catalysed air-oxidation of acyloins. J. Chem. Soc. 1951, 1924–1925. [Google Scholar] [CrossRef]
- Rigby, W. A new reagent for the oxidation of acyloins to diketones. J. Chem. Soc. 1951, 793–795. [Google Scholar] [CrossRef]
- Djerassi, C.; Ringold, H.J.; Rosenkranz, G. Steroidal sapogenins. 15. Experiments in the hecogenin series (Part 3). Conversion to cortisone. J. Am. Chem. Soc. 1951, 73, 5513–5514. [Google Scholar] [CrossRef]
- Djerassi, C.; Ringold, H.J.; Rosenkranz, G. Steroidal sapogenins. 37. Experiments in the hecogenin series (Part 6). Conversion to cortisone. J. Am. Chem. Soc. 1954, 76, 5533–5536. [Google Scholar] [CrossRef]
- Becker, E.J.; Cohen, A. 12-Keto progestational steroids and derivatives thereof. US Patent 3,185,713, 25 May 1965. [Google Scholar]
- Kurath, P. Structure and stereochemistry of benzilic acid rearrangement product of 3α,17β-diacetoxy-11-Hydroxy-5β-androst-9(11)-en-12-one. J. Org. Chem. 1967, 32, 3626–3635. [Google Scholar] [CrossRef]
- Baran, J.S. 2-Hydroxy-Δ1,4-3-keto steroids. J. Am. Chem. Soc. 1958, 80, 1687–1691. [Google Scholar] [CrossRef]
- Chaudhry, G.R.; Halsall, T.G.; Jones, E.R.H. Chemistry of triterpenes and related compounds. 39. Some derivatives of 4,4-dimethylcholestan-3-one. J. Chem. Soc. 1961, 2725–2732. [Google Scholar]
- Marsh, D.A.; Brodie, H.J.; Garrett, W.; Tsaimorris, C.H.; Brodie, A.M.H. Aromatase inhibitors. Synthesis and biological activity of androstenedione derivatives. J. Med. Chem. 1985, 28, 788–795. [Google Scholar] [CrossRef]
- Agnello, E.J.; Laubach, G.D.; Figdor, S.K.; Pinson, R.; Ordway, H.W.; Bloom, B.M.; Hughes, G.M.K. A stereospecific synthesis of C-21-methylated corticosteroids. J. Org. Chem. 1963, 28, 1531–1539. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Lavie, D. Schoenocaulon alkaloids. III. The bismuth oxide oxidation of veracevine, cevagenine and cevine. J. Am. Chem. Soc. 1955, 77, 683–686. [Google Scholar] [CrossRef]
- Reitsema, R.H. The Synthesis of Racemic Piperitenone Oxide and Diosphenolene. J. Am. Chem. Soc. 1957, 79, 4465–4468. [Google Scholar] [CrossRef]
- Corey, E.J.; Tius, M.A.; Das, J. Total synthesis of (+/-)-aphidicolin. J. Am. Chem. Soc. 1980, 102, 1742–1744. [Google Scholar] [CrossRef]
- Lavie, D.; Shvo, Y. Constituents od Ecballium elaterium L.: Proposed structures for elatericin A and elatericin B. Chem. Ind. 1959, 429–430. [Google Scholar]
- Lavie, D.; Shvo, Y.; Willner, D.; Enslin, P.R.; Hugo, J.M.; Norton, K.B. Interrelationships in the cucurbitacin series. Chem. Ind. 1959, 951–952. [Google Scholar]
- Sasaki, M.; Murae, T.; Takahashi, T. Synthesis of (+/-)-15-deoxybruceolide and conversion of (-)-15-deoxybruceolide into (-)-bruceantin: Total synthesis of bruceantin. J. Org. Chem. 1990, 55, 528–540. [Google Scholar] [CrossRef]
- Parish, E.J.; Kizito, S.A.; Qiu, Z. Review of chemical synthesis of 7-keto-Δ5-sterols. Lipids 2004, 39, 801–804. [Google Scholar]
- Salvador, J.A.R.; Silvestre, S.M.; Moreira, V.M. Catalytic oxidative processes in steroid chemistry: Allylic oxidation, β-selective epoxidation, alcohol oxidation and remote functionalization reactions. Curr. Org. Chem. 2006, 10, 2227–2257. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Silvestre, S.M. Bismuth-catalyzed allylic oxidation using t-butyl hydroperoxide. Tetrahedron Lett. 2005, 46, 2581–2584. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Silvestre, S.M. Processo para a oxidação alílica de compostos insaturados usando hidroperóxido de t-butilo e catalisadores de bismuto. PT Patent 103,211, 15 September 2006. [Google Scholar]
- Salvador, J.A.R.; Silvestre, S.M. Faculty of Pharmacy. University of Coimbra: Portugal, 2006; Unpublished results. [Google Scholar]
- Samadjar, S.; Becker, F.F.; Banik, B.K. Surface-mediated highly efficient oxidation of alcohols by bismuth nitrate. Synth. Commun. 2001, 31, 2691–2695. [Google Scholar] [CrossRef]
- Reese, A.L.; McMartin, K.; Miller, D.; Wickham, P.P. Reactions of bismuth triacetate with organic compounds. J. Org. Chem. 1973, 38, 764–768. [Google Scholar]
- Orita, A.; Tanahashi, C.; Kakuda, A.; Otera, J. Highly efficient and versatile acylation of alcohols with Bi(OTf)3 as catalyst. Angew. Chem. Int. Ed. 2000, 39, 2877–2879. [Google Scholar] [CrossRef]
- Orita, A.; Tanahashi, C.; Kakuda, A.; Otera, J. Highly powerful and practical acylation of alcohols with acid anhydride catalyzed by Bi(OTf)3. J. Org. Chem. 2001, 66, 8926–8934. [Google Scholar] [CrossRef]
- Carrigan, M.D.; Freiberg, D.A.; Smith, R.C.; Zerth, H.M.; Mohan, R.S. A simple and practical method for large-scale acetylation of alcohols and diols using bismuth triflate. Synthesis 2001, 2091–2094. [Google Scholar]
- Mohammadpoor-Baltork, I.; Aliyan, H.; Khosropour, A.R. Bismuth(III) salts as convenient and efficient catalysts for the selective acetylation and benzoylation of alcohols and phenols. Tetrahedron 2001, 57, 5851–5854. [Google Scholar] [CrossRef]
- Mohammadpoor-Baltork, I.; Khosropour, A.R.; Aliyan, H. A convenient and chemoselective acetylation and formylation of alcohols and phenols using acetic acid and ethyl formate in the presence of Bi(III) salts. J. Chem. Res. (M) 2001, 280–282. [Google Scholar]
- Ghosh, R.; Maiti, S.; Chakraborty, A. Facile catalyzed acylation of heteroatoms using BiCl3 generated in situ from the procatalyst BiOCl and acetyl chloride. Tetrahedron Lett. 2004, 45, 6775–6778. [Google Scholar] [CrossRef]
- Mohammadpoor-Baltork, I.; Khosropour, A.R. Bi(III) salts as new catalysts for the selective conversion of trimethylsilyl and tetrahydropyranyl ethers to their corresponding acetates and formates. Synth. Commun. 2002, 32, 2433–2439. [Google Scholar] [CrossRef]
- Mohammadpoor-Baltork, I.; Khosropour, A.R. Efficient and selective conversion of trimethylsilyl and tetrahydropyranyl ethers to their corresponding acetates and benzoates catalyzed by bismuth(III) salts. Mon. Chem. 2002, 133, 189–193. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Pinto, R.M.A.; Santos, R.C.; Le Roux, C.; Beja, A.M.; Paixao, J.A. Bismuth triflate-catalyzed Wagner-Meerwein rearrangement in terpenes. Application to the synthesis of the 18α-oleanane core and A-neo-18α-oleanene compounds from lupanes. Org. Biomol. Chem. 2009, 7, 508–517. [Google Scholar] [CrossRef]
- Stephens, J.R.; Butler, P.L.; Clow, C.H.; Oswald, M.C.; Smith, R.C.; Mohan, R.S. Bismuth triflate: An efficient catalyst for the formation and deprotection of tetrahydropyranyl ethers. Eur. J. Org. Chem. 2003, 3827–3831. [Google Scholar]
- Khan, A.T.; Ghosh, S.; Choudhury, L.H. A highly efficient synthetic protocol for tetrahydropyranylation/depyranylation of alcohols and phenols. Eur. J. Org. Chem. 2005, 4891–4896. [Google Scholar]
- Keramane, E.M.; Boyer, B.; Roque, J.-P. BiBr3-catalyzed benzylation of alcohols. Stereochemistry and mechanistic investigations. Tetrahedron 2001, 57, 1917–1921. [Google Scholar] [CrossRef]
- Kadam, S.T.; Kim, S.S. Mild and efficient silylation of alcohols and phenols with HMDS using Bi(OTf)3 under solvent-free condition. J. Organomet. Chem. 2009, 694, 2562–2566. [Google Scholar] [CrossRef]
- Carrigan, M.D.; Sarapa, D.; Smith, R.C.; Wieland, L.C.; Mohan, R.S. A simple and efficient chemoselective method for the catalytic deprotection of acetals and ketals using bismuth triflate. J. Org. Chem. 2002, 67, 1027–1030. [Google Scholar] [CrossRef]
- Bailey, A.D.; Ashvin, R.B.; Tasche, K.K.; Mohan, R.S. Environmentally friendly organic synthesis using bismuth compounds: Bismuth(III) iodide catalyzed deprotection of acetals. Tetrahedron Lett. 2008, 49, 691–694. [Google Scholar]
- Mohammadpoor-Baltork, I.; Kharamesh, B.; Kolagar, S. Efficient deprotection of tetrahydropyranyl ethers by bismuth(III) salts. Synth. Commun. 2002, 32, 1633–1637. [Google Scholar] [CrossRef]
- Sabitha, G.; Babu, R.S.; Reddy, E.V.; Srividya, R.; Yadav, J.S. A novel, efficient, and selective cleavage of alkyl tert-butyldimethylsilyl ethers using BiCl3/NaI system. Adv. Synth. Catal. 2001, 343, 169–170. [Google Scholar] [CrossRef]
- Boruah, A.; Baruah, B.; Prajapati, D.; Sandhu, J.S. Regeneration of carbonyl compounds from oximes under microwave irradiations. Tetrahedron Lett. 1997, 38, 4267–4268. [Google Scholar]
- Pinto, R.M.A.; Salvador, J.A.R.; Le Roux, C. Ritter reaction mediated by bismuth(III) salts: One-step conversion of epoxides into vic-acylamino-hydroxy compounds. Synlett 2006, 2047–2050. [Google Scholar]
- Pinto, R.M.A.; Silva, M.R.; Beja, A.M.; Salvador, J.A.R.; Paixão, J.A. 5α-Acetamido-6β-hydroxy-17-oxoandrostan-3β-yl acetate. Acta Crystallogr. Sect. E 2007, E63, o3321. [Google Scholar]
- Pinto, R.M.A.; Silva, M.R.; Beja, A.M.; Salvador, J.A.R.; Paixao, J.A. 6β-Acetamido-5α-hydroxycholestan-3β-yl acetate. Acta Crystallogr. Sect. E 2008, E64, o2303. [Google Scholar]
- Sanderson, J.; Bayse, C.A. The Lewis acidity of bismuth(III) halides: A DFT analysis. Tetrahedron 2008, 64, 7685–7689. [Google Scholar] [CrossRef]
- Pinto, R.M.A.; Salvador, J.A.R.; Le Roux, C. Bismuth(III) salts mediated regioselective ring opening of epoxides: An easy route to halohydrins and β-hydroxy nitrates. Tetrahedron 2007, 63, 9221–9228. [Google Scholar]
- Pinto, R.M.A.; Salvador, J.A.R.; Paixao, J.A.; Beja, A.M.; Silva, M.R. 6β-Chloro-5α-hydroxy-20-oxopregnan-3β-yl acetate. Acta Crystallogr. Sect. E 2008, E64, o1420. [Google Scholar]
- Pinto, R.M.A.; Matos Beja, A.; Salvador, J.A.R.; Paixão, J.A. 16α,17α-Epoxy-5α-hydroxy-6β-nitrooxy-20-oxopregnan-3β-yl acetate. Acta Crystallogr. Sect. E 2009, E65, o1271–o1272. [Google Scholar]
- Wabnitz, T.C.; Yu, J.Q.; Spencer, J.B. Evidence that protons can be the active catalysts in Lewis acid mediated hetero-Michael addition reactions. Chem. Eur. J. 2004, 10, 484–493. [Google Scholar] [CrossRef]
- Keramane, E.M.; Boyer, B.; Rogue, J.P. Reactivity of bismuth(III) halides towards alcohols. A tentative to mechanistic investigation. Tetrahedron 2001, 57, 1909–1916. [Google Scholar] [CrossRef]
- Carvalho, J.F.S.; Silva, M.M.C.; Sá e Melo, M.L. Highly efficient epoxidation of unsaturated steroids using magnesium bis(monoperoxyphathalate) hexahydrate. Tetrahedron 2009, 65, 2773–2781. [Google Scholar]
- Carvalho, J.F.S.; Silva, M.M.C.; Melo, M. Efficient trans-diaxial hydroxylation of Δ5-steroids. Tetrahedron 2010, 66, 2455–2462. [Google Scholar]
- Carvalho, J.F.S.; Silva, M.M.C.; Moreira, J.N.; Simoes, S.; Melo, M.L.S. Sterols as anticancer agents: Synthesis of Ring-B oxygenated steroids, cytotoxic profile, and comprehensive SAR analysis. J. Med. Chem. 2010, 53, 7632–7638. [Google Scholar] [CrossRef]
- Swamy, N.R.; Venkateswarlu, Y. An efficient method for the synthesis of 2,3-unsaturated glycopyranosides catalyzed by bismuth trichloride in Ferrier rearrangement. Synthesis 2002, 598–600. [Google Scholar]
- Babu, J.L.; Khare, A.; Vankar, Y.D. Bi(OTf)3 and SiO2-Bi(OTf)3 as effective catalysts for the Ferrier rearrangement. Molecules 2005, 10, 884–892. [Google Scholar] [CrossRef]
- Lacey, J.R.; Anzalone, P.W.; Duncan, C.M.; Hackert, M.J.; Mohan, R.S. A study of epoxyolefin cyclizations catalyzed by bismuth trifluoromethanesulfonate and other metal triflates. Tetrahedron Lett. 2005, 46, 8507–8511. [Google Scholar] [CrossRef]
- Smith, B.M.; Skellam, E.J.; Oxley, S.J.; Graham, A.E. Highly selective synthesis of oxabicycloalkanes by indium tribromide-mediated cyclization reactions of epoxyalkenes. Org. Biomol. Chem. 2007, 5, 1979–1982. [Google Scholar] [CrossRef]
- Pinto, R.M.A.; Salvador, J.A.R.; Le Roux, C.; Carvalho, R.A.; Silva, M.R.; Beja, A.M.; Paixão, J.A. Bismuth(III) salt-catalyzed Westphalen and "backbone" rearrangements of 5β,6β-epoxysteroids. Synthesis and structural elucidation of new olefinic 19-nor and 18,19-dinorsteroids. Steroids 2008, 73, 549–561. [Google Scholar] [CrossRef]
- Pinto, R.M.A.; Ramos Silva, M.; Matos Beja, A.; Salvador, J.A.R.; Paixão, J.A. 6β-Hydroxy-5β-methyl-20-oxo-19-norpregn-9(10)-en-3β-yl acetate. Acta Crystallogr. Sect. C 2009, C65, o214–o216. [Google Scholar]
- Pinto, R.M.A.; Salvador, J.A.R.; Paixão, J.A. 5β,6β-Epoxy-17-oxoandrostan-3β-yl acetate and 5β,6β-epoxy-20-oxopregnan-3β-yl acetate. Acta Crystallogr. Sect. C 2008, C64, o279–o282. [Google Scholar]
- into, R.M.A.; Salvador, J.A.R.; Le Roux, C.; Carvalho, R.A.; Beja, A.M.; Paixão, J.A. Bismuth(III) triflate-catalyzed rearrangement of 16α,17α-epoxy-20-oxosteroids. Synthesis and structural elucidation of new 16α-substituted 17α-alkyl-17β-methyl-Δ13-18-norsteroids. Tetrahedron 2009, 65, 6169–6178. [Google Scholar] [CrossRef]
- Hanson, J.R. Wagner-Meerwein rearrangements. In Comprehensive Organic Synthesis; Pattenden, G., Ed.; Pergamon Press plc: Oxford, UK, 1991; Volume 3, pp. 705–719. [Google Scholar]
- Santos, R.C.; Pinto, R.M.A.; Matos Beja, A.; Salvador, J.A.R.; Paixão, J.A. 19β,28-Epoxy-18α-olean-3β-ol. Acta Crystallogr. Sect. E 2009, E65, o2088–o2089. [Google Scholar]
- Repichet, S.; Zwick, A.; Vendier, L.; Le Roux, C.; Dubac, J. A practical, cheap and environmentally friendly preparation of bismuth(III) trifluoromethanesulfonate. Tetrahedron Lett. 2002, 43, 993–995. [Google Scholar]
- Vatèle, J.-M. Lewis acid promoted oxidative rearrangement of tertiary allylic alcohols with the PhIO/TEMPO System. Synlett 2008, 1785–1788. [Google Scholar]
- Vatèle, J.-M. Lewis acid-catalyzed oxidative rearrangement of tertiary allylic alcohols mediated by TEMPO. Tetrahedron 2010, 66, 913–917. [Google Scholar] [CrossRef]
- Kiesman, W.F.; Petter, R.C. Lewis-acid catalysis of the asymmetric Diels-Alder reaction of dimenthyl fumarate and cyclopentadiene. Tetrahedron Asymm. 2002, 13, 957–960. [Google Scholar] [CrossRef]
- Peidro, L.; Le Roux, C.; Laporterie, A.; Dubac, J. BiCl3-catalyzed Mukaiyama-aldol and carbonyl-ene reactions. J. Organomet. Chem. 1996, 521, 397–399. [Google Scholar] [CrossRef]
- Anderson, E.D.; Ernat, J.J.; Nguyen, M.P.; Palma, A.C.; Mohan, R.S. Environment friendly organic synthesis using bismuth compounds. An efficient method for carbonyl-ene reactions catalyzed by bismuth triflate. Tetrahedron Lett. 2005, 46, 7747–7750. [Google Scholar] [CrossRef]
- Samajdar, S.; Becker, F.F.; Banik, B.K. Surface-mediated highly efficient regioselective nitration of aromatic compounds by bismuth nitrate. Tetrahedron Lett. 2000, 41, 8017–8020. [Google Scholar] [CrossRef]
- Samajdar, S.; Becker, F.F.; Banik, B.K. Montmorillonite impregnated with bismuth nitrate: A versatile reagent for the synthesis of nitro compounds of biological significance. ARKIVOC 2001, viii, 27–33. [Google Scholar]
- Bose, A.; Sanjoto, W.P.; Villarreal, S.; Aguilar, H.; Banik, B.K. Novel nitration of estrone by metal nitrates. Tetrahedron Lett. 2007, 48, 3945–3947. [Google Scholar] [CrossRef]
- Santos, R.C.; Pinto, R.M.A.; Matos Beja, A.; Salvador, J.A.R.; Paixão, J.A. 3-Oxo-18α-olean-28,13β-olide. Acta Crystallogr. Sect. E 2010, E66, o2139–o2140. [Google Scholar]
- Pinto, R.M.A.; Salvador, J.A.R.; Le Roux, C.; Paixão, J.A. Bismuth(III) triflate-catalyzed direct conversion of corticosteroids into highly functionalized 17-ketosteroids by cleavage of the C17-dihydroxyacetone side chain. J. Org. Chem. 2009, 74, 8488–8491. [Google Scholar] [CrossRef]
- Oliveto, E.P. Synthesis and degradation of the pregnane side-chain. In Organic Reactions in Steroid Chemistry; Fried, J., Edwards, J.A., Eds.; Van Nostrand Reinhold Company: New York, NY, USA, 1972; Volume 2, pp. 127–236. [Google Scholar]
- Le Pera, A.; Leggio, A.; Siciliano, C.; Di Gioia, M.L.; Napoli, A.; Sindona, G.; Liguori, A. A straightforward chemical synthesis of 17-ketosteroids by cleavage of the C-17-dihydroxy acetone side chain in corticosteroids. Steroids 2003, 68, 139–142. [Google Scholar] [CrossRef]
- Sun, L.; Geng, X.; Liu, L.H.; Jiang, C.G.; Wang, C. Iodine-promoted cleavage of the C-17-dihydroxyacetone side chain of corticosteroids in aqueous ammonia water. J. Chem. Res. (M) 2009, 22–23. [Google Scholar]
- Avery, M.A.; Woolfrey, J.R. Anti-inflammatory steroids. In Burger's Medicinal Chemistry and Drug Discover: Cardiovascular Agents and Endrocrines; Abraham, D.J., Ed.; John Wiley & Sons, Inc: New York, NY, USA, 2003; Volume 3, pp. 747–853. [Google Scholar]
- McGhie, S.; Strachan, C.; Aitken, S. A review of the use of aqueous hydrofluoric acid in the manufacture of betamethasone. Org. Process Res. Dev. 2002, 6, 898–900. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Salvador, J.A.R.; Silvestre, S.M.; Pinto, R.M.A. Bismuth(III) Reagents in Steroid and Terpene Chemistry. Molecules 2011, 16, 2884-2913. https://doi.org/10.3390/molecules16042884
Salvador JAR, Silvestre SM, Pinto RMA. Bismuth(III) Reagents in Steroid and Terpene Chemistry. Molecules. 2011; 16(4):2884-2913. https://doi.org/10.3390/molecules16042884
Chicago/Turabian StyleSalvador, Jorge A. R., Samuel M. Silvestre, and Rui M. A. Pinto. 2011. "Bismuth(III) Reagents in Steroid and Terpene Chemistry" Molecules 16, no. 4: 2884-2913. https://doi.org/10.3390/molecules16042884



