Microwave-Assisted Solution-Phase Synthesis and DART-Mass Spectrometric Monitoring of a Combinatorial Library of Indolin-2,3-dione Schiff Bases with Potential Antimycobacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

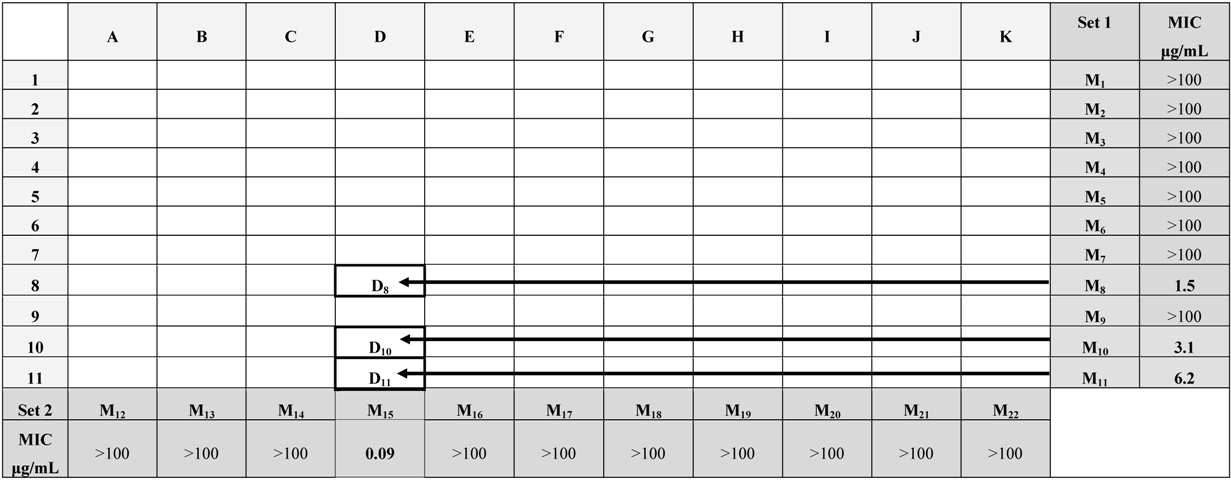
 | A | B | C | D | E | F | G | H | I | J | K | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
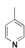 |  | 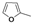 | 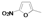 |  | 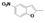 |  | 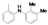 | 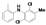 |  |  | Set. 1 | ||
| 1 | 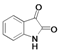 | A1 | B1 | C1 | D1 | E1 | F1 | G1 | H1 | I1 | J1 | K1 | M1 |
| 2 | 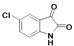 | A2 | B2 | C2 | D2 | E2 | F2 | G2 | H2 | I2 | J2 | K2 | M2 |
| 3 |  | A3 | B3 | C3 | D3 | E3 | F3 | G3 | H3 | I3 | J3 | K3 | M3 |
| 4 | 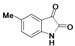 | A4 | B4 | C4 | D4 | E4 | F4 | G4 | H4 | I4 | J4 | K4 | M4 |
| 5 | 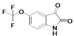 | A5 | B5 | C5 | D5 | E5 | F5 | G5 | H5 | I5 | J5 | K5 | M5 |
| 6 | 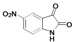 | A6 | B6 | C6 | D6 | E6 | F6 | G6 | H6 | I6 | J6 | K6 | M6 |
| 7 |  | A7 | B7 | C7 | D7 | E7 | F7 | G7 | H7 | I7 | J7 | K7 | M7 |
| 8 |  | A8 | B8 | C8 | D8 | E8 | F8 | G8 | H8 | I8 | J8 | K8 | M8 |
| 9 |  | A9 | B9 | C9 | D9 | E9 | F9 | G9 | H9 | I9 | J9 | K9 | M9 |
| 10 |  | A10 | B10 | C10 | D10 | E10 | F10 | G10 | H10 | I10 | J10 | K10 | M10 |
| 11 |  | A11 | B11 | C11 | D11 | E11 | F11 | G11 | H11 | I11 | J11 | K11 | M11 |
| Set 2 | M12 | M13 | M14 | M15 | M16 | M17 | M18 | M19 | M20 | M21 | M22 | ||
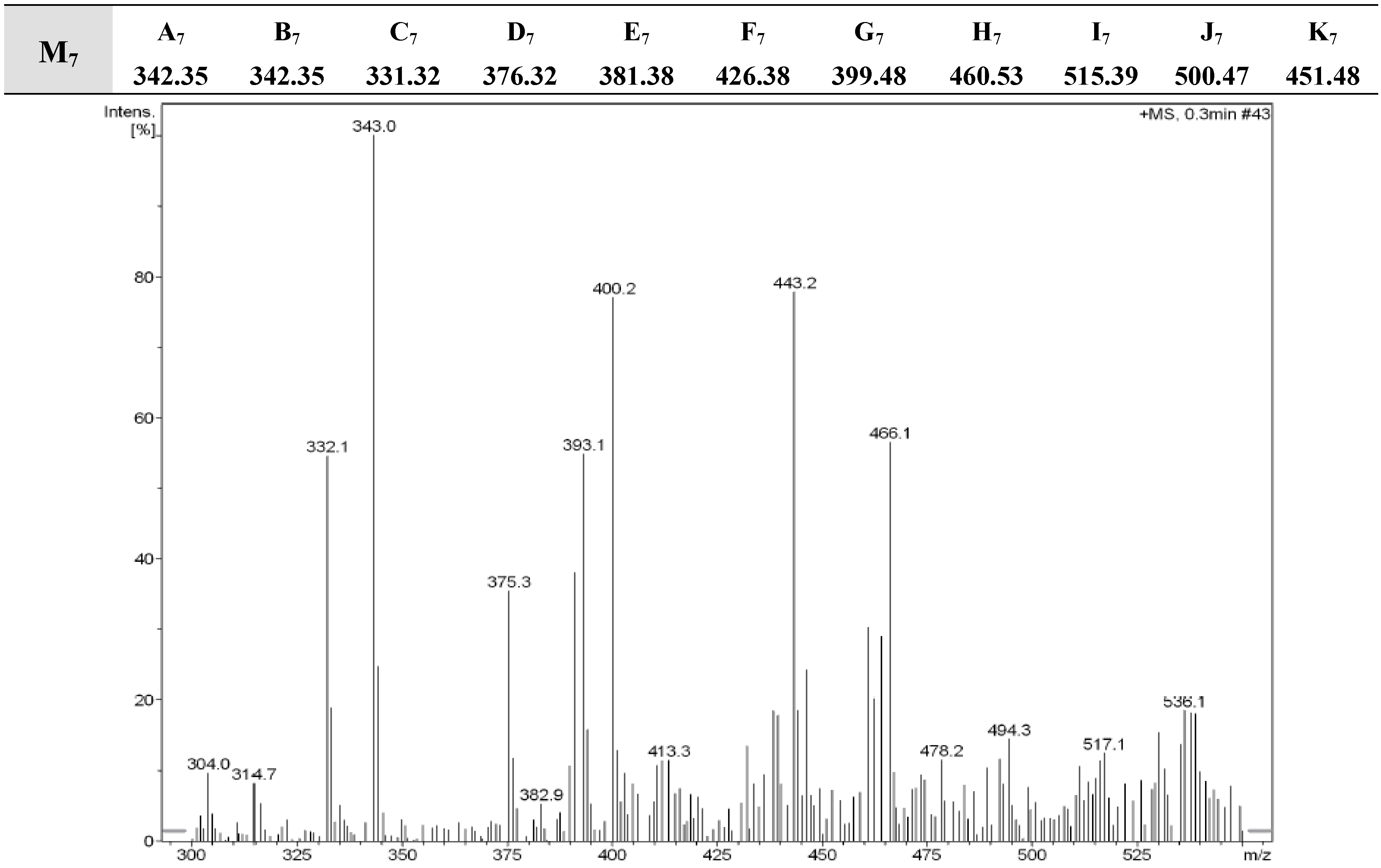

2.2. Antimycobacterial Activity
3. Experimental
3.1. General
3.2. Preparation of Combinatorial Mixtures
3.2.1. Synthesis of Schiff base mixtures M1-M11 (set. 1)
3.2.2. Synthesis of Schiff base mixtures M12-M22 (set. 2)
3.3. DART-Spectrometric Analysis
3.4. Evaluation of Antimycobacterial Activity of the Synthesized Library
4. Conclusions
Acknowledgements
References
- World Health Organization. WHO: “Tuberculosis” Fact sheet No. 104. November 2010. Available online: http://www.who.int/mediacentre/factsheets/fs104/en/index.html (accessed on 4 May 2011).
- Dye, C.; Williams, B.G. The Population Dynamics and Control of Tuberculosis. Science 2010, 328, 856–861. [Google Scholar] [CrossRef]
- WHO Progress Report 2011. Available online: http://www.who.int/tb/en/ (accessed on 4 May 2011).
- De Souza, M.V.N. Promising drugs against tuberculosis. Recent Pat. Anti-Infect. Drug Discov. 2006, 1, 33–44. [Google Scholar]
- De Souza, M.V.N. Current status and future prospects for new therapies for pulmonary tuberculosis. Curr. Opin. Pulm. Med. 2006, 12, 167–171. [Google Scholar] [CrossRef]
- Junior, I.N.; Lourenço, M.C.S.; Henriques, M.G.M.O.; Ferreira, B.; Vasconcelos, T.R.A.; Peralta, M.A.; De Oliveira, P.S.M.; Wardell, S.M.S.V.; De Souza, M.V.N. Synthesis and anti-Mycobacterial activity of N'-[(E)-(Disubstituted-Phenyl)methylidene]isoni-cotino-hydrazide derivatives. Lett. Drug Des. Dis. 2005, 2, 563–566. [Google Scholar]
- Ferreira, M.L.; Vasconcelos, T.R.A.; de Carvalho, E.M.; Lourenço, M.C.S.; Wardell, S.M.S.V.; Wardell, J.L.; Ferreira, V.F.; de Souza, M.V.N. Synthesis and antitubercular activity of novel Schiff bases derived from D-mannitol. Carbohyd. Res. 2009, 344, 2042–2047. [Google Scholar] [CrossRef]
- Carvalho, S.A.; da Silva, E.F.; de Souza, M.V.N.; Lourenço, M.C.S.; Vicente, F.R. Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 538–541. [Google Scholar] [CrossRef]
- Lourenço, M.C.S.; Ferreira, M.L.; De Souza, M.V.N.; Peralta, M.A.; Vasconcelos, T.R.A.; Henriques, M.G.M.O. Synthesis and anti-mycobacterial activity of (E)-N′-(monosubstituted-benzylidene)isonicotinohydrazide derivatives. Eur. J. Med. Chem. 2008, 43, 1344–1347. [Google Scholar] [CrossRef]
- Hearn, M.J.; Cynamon, M.H.; Chen, M.F.; Coppins, R.; Davis, J.; Joo-On Kang, H.; Noble, A.; Tu-Sekine, B.; Terrot, M.S.; Trombino, D.; et al. Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. Eur. J. Med. Chem. 2009, 44, 4169–4178. [Google Scholar] [CrossRef]
- Lourenço, M.C.; De Souza, M.V.N.; Pinheiro, A.C.; Ferreira, M.L.; Gonçalves, R.S.B.; Nogueira, T.C.M.; Peralta, M.A. Evaluation of anti-tubercular activity of nicotinic and isoniazid analogues. ARKIVOC 2007, XV, 181–191. [Google Scholar]
- Aboul-Fadl, T.; Bin-Jubair, F.A.S.; Aboul-Wafa, O. Schiff bases of indoline-2,3-dione (isatin) derivatives and nalidixic acid carbohydrazide, synthesis, antitubercular activity and pharmacophoric model building. Eur. J. Med. Chem. 2010, 45, 4578–4586. [Google Scholar] [CrossRef]
- Abdel-Aal, W.S.; Hassan, H.Y.; Aboul-Fadl, T.; Youssef, A.F. Pharmacophoric model building for antitubercular activity of the individual Schiff bases of small combinatorial library. Eur. J. Med. Chem. 2010, 45, 1098–1106. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Mohammed, F.A.; Hassan, E.A. Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff bases derived from 1-alkylisatin and isonicotinic acid hydrazide (INH). Arch. Pharm. Res. 2003, 26, 778–784. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Bin-Jubair, F.A.S. Anti-tubercular activity of isatin derivatives. Int. J. Res. Pharm. Sci. 2010, 1, 113–126. [Google Scholar]
- Pathak, R.; Pant, C.S.; Shaw, A.K.; Bhaduri, A.P.; Gaikwad, A.N.; Sinha, S.; Srivastava, A.; Srivastava, K.K.; Chaturvedi, V.; Srivastavac, R.; Srivastava, B.S. Baylis–Hillman reaction: Convenient ascending syntheses and biological evaluation of acyclic deoxy monosaccharides as potential antimycobacterial agents. Bioorg. Med. Chem. 2002, 10, 3187–3196. [Google Scholar]
- Drews, J. Drug discovery: A historical perspective. Science 2000, 287, 1960–1964. [Google Scholar] [CrossRef]
- Baudry, J.; Hergenrother, P.J. Structure-based design and in silico virtual screening of combinatorial libraries. A combined chemical computational project. J. Chem. Educ. 2005, 82, 890–894. [Google Scholar] [CrossRef]
- Dolle, R.E.; Le Bourdonnec, B.; Goodman, A.J.; Morales, G.A.; Salvino, J.M.; Zhang, W. Comprehensive survey of combinatorial libraries for drug discovery and chemical biology. J. Comb. Chem. 2007, 9, 855–902. [Google Scholar] [CrossRef]
- Cheng, S.; Comer, D.D.; Williams, J.P.; Myers, P.L.; Boger, D. Novel solution phase strategy for the synthesis of chemical libraries containing small organic molecules. J. Am. Chem. Soc. 1996, 118, 2567–2573. [Google Scholar] [CrossRef]
- Bhatia, N.M.; Mahadik, K. Solution phase combinatorial synthesis and screening of mini libraries of arylchalcones for antibacterial activity. Sci. Pharm. 2008, 76, 259–267. [Google Scholar] [CrossRef]
- Abdel-Aal, W.S.; Hassan, H.Y.; Aboul-Fadl, T.; Youssef, A.F. Solution-phase synthesis of small Schiff bases combinatorial library with potential antitubercular activity. Der Pharma Chemica 2009, 1, 1–13. [Google Scholar]
- Kappe, C.O.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug. Discov. 2006, 5, 51–63. [Google Scholar]
- Kappe, C.O.; Dallinger, D. Controlled microwave heating in modern organic synthesis: Highlights from the 2004–2008 literature. Mol. Divers. 2009, 13, 71–193. [Google Scholar] [CrossRef]
- Kappe, C.O. Microwave dielectric heating in synthetic organic chemistry. Chem. Soc. Rev. 2008, 37, 1127–1139. [Google Scholar]
- Moseley, J.D.; Kappe, C.O.A. Critical assessment of the greenness and energy efficiency of microwave-assisted organic synthesis. Green Chem. 2011, 13, 794–806. [Google Scholar] [CrossRef]
- Bose, A.K.; Manhas, M.S.; Banik, B.K.; Robb, E.W. Microwave-induced organic reaction enhancement (more) chemistry: Techniques for rapid, safe and inexpensive synthesis. Res. Chem. Intermed. 1994, 20, 1–11. [Google Scholar]
- Cody, R.B.; Laramée, J.A.; Durst, H.D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef]
- Petucci, C.; Diffendal, J.; Kaufman, D.; Mekonnen, B.; Terefenko, G.; Musselman, B. Direct analysis in real time for reaction monitoring in drug discovery. Anal. Chem. 2007, 79, 5064–5070. [Google Scholar]
- Kappe, C.O. High-speed combinatorial synthetics utilizing microwave irradiation. Curr. Opin. Chem. Biol. 2002, 6, 314–320. [Google Scholar]
- Loughlin, W.A.; Marshall, R.L.; Carriro, A.; Elson, K.E. Solution-Phase Combinatorial Synthesis and Evaluation of Piperazine-2,5-dione Derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 91–94. [Google Scholar] [CrossRef]
- Chang, J.; Zhao, K.; Pan, S. Synthesis of 2-arylbenzoxazoles via DDQ promoted oxidative cyclization of phenolic Schiff bases a solution-phase strategy for library synthesis. Tetrahydron Lett. 2002, 43, 951–954. [Google Scholar] [CrossRef]
- Wolkenberg, S.E.; Su, A.I. Combinatorial synthesis and discovery of an antibiotic compound. J. Chem. Educ. 2001, 78, 784–785. [Google Scholar] [CrossRef]
- Bajpai, V.; Sharma, D.; Kumar, B.; Madhusudanan, K.P. Profiling of Piper betle Linn. cultivars by direct analysis in real time mass spectrometric technique. Biomed. Chromatogr. 2010, 24, 1283–1286. [Google Scholar] [CrossRef]
- Vaclavik, L.; Cajka, T.; Hrbek, V.; Hajslova, J. Ambient mass spectrometry employing direct analysis in real time (DART) ion source for olive oil quality and authenticity assessment. Anal. Chim. Acta 2009, 645, 56–63. [Google Scholar] [CrossRef]
- Gu, H.; Pan, Z.; Xi, B.; Asiago, V.; Musselman, B.; Raftery, D. Principal component directed partial least squares analysis for combining nuclear magnetic resonance and mass spectrometry data in metabolomics: Application to the detection of breast cancer. Anal. Chim. Acta 2011, 686, 57–63. [Google Scholar] [CrossRef]
- Edison, S.E.; Lin, L.A.; Gamble, B.M.; Wong, J.; Zhang, K. Surface swabbing technique for the rapid screening for pesticides using ambient pressure desorption ionization with high-resolution mass spectrometry. Rapid Comm. Mass Spec. 2011, 25, 127–139. [Google Scholar] [CrossRef]
- Zhou, M.; McDonald, J.F.; Fernández, F.M. Optimization of a direct analysis in real time/time-of-flight mass spectrometry method for rapid serum metabolomic fingerprinting. J. Am. Soc. Mass Spec. 2010, 21, 68–75. [Google Scholar] [CrossRef]
- Vaclavik, L.; Zachariasova, M.; Hrbek, V.; Hajslova, J. Analysis of multiple mycotoxins in cereals under ambient conditions using direct analysis in real time (DART) ionization coupled to high resolution mass spectrometry. Talanta 2010, 82, 1950–1957. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Abdel-aziz, H.A.; Kadi, A.; Bari, A.; Ahmad, A.; Al-samani, T.; Weng Ng, S. Microwave-assisted, one-step synthesis of fenamic acid hydrazides from the corresponding acids. Molecules 2011, 16, 3544–3551. [Google Scholar] [CrossRef]
- El-Emam, A.A.; Moustafa, M.A.; Abdelal, A.M.; El-Ashmawy, M.B. Triazoles and Fused Triazoles IV: Synthesis of 3-(1-adamantyl)-6-substituted-s-triazolo[3,4-b][1,3,4]thiadiazoles and 3-(1-adamantyl)-6-aryl-7H-s-triazolo[3,4-b][1,3,4]thia-diazines as potential chemotherapeutic agents. Chin. Pharm. J. 1993, 45, 101–107. [Google Scholar]
- Canetti, G.; Rist, N.; Grosset, J. Measurement of sensitivity of the tuberculous bacillus to antibacillary drugs by the method of proportions. Methodology, resistance criteria, results and interpretation. Rev. Tuberc. Pneumol. (Paris) 1963, 27, 217–272. [Google Scholar]
- Sample Availability: Samples of the compounds are available from Prof. Tarek Aboul-Fadl, Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aboul-Fadl, T.; Abdel-Aziz, H.A.; Kadi, A.; Ahmad, P.; Elsaman, T.; Attwa, M.W.; Darwish, I.A. Microwave-Assisted Solution-Phase Synthesis and DART-Mass Spectrometric Monitoring of a Combinatorial Library of Indolin-2,3-dione Schiff Bases with Potential Antimycobacterial Activity. Molecules 2011, 16, 5194-5206. https://doi.org/10.3390/molecules16065194
Aboul-Fadl T, Abdel-Aziz HA, Kadi A, Ahmad P, Elsaman T, Attwa MW, Darwish IA. Microwave-Assisted Solution-Phase Synthesis and DART-Mass Spectrometric Monitoring of a Combinatorial Library of Indolin-2,3-dione Schiff Bases with Potential Antimycobacterial Activity. Molecules. 2011; 16(6):5194-5206. https://doi.org/10.3390/molecules16065194
Chicago/Turabian StyleAboul-Fadl, Tarek, Hatem A. Abdel-Aziz, Adnan Kadi, Pervez Ahmad, Tilal Elsaman, Mohamed W. Attwa, and Ibrahim A. Darwish. 2011. "Microwave-Assisted Solution-Phase Synthesis and DART-Mass Spectrometric Monitoring of a Combinatorial Library of Indolin-2,3-dione Schiff Bases with Potential Antimycobacterial Activity" Molecules 16, no. 6: 5194-5206. https://doi.org/10.3390/molecules16065194