Expeditious Entry to Novel 2-Methylene-2,3-dihydrofuro[3,2-c] chromen-2-ones from 6-Chloro-4-hydroxychromen-2-one and Propargylic Alcohols
Abstract
:1. Introduction



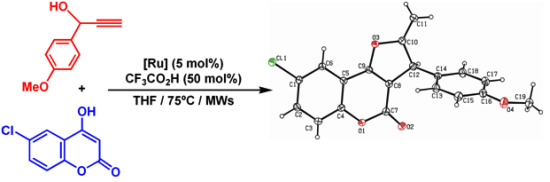
2. Results and Discussion


| Distances | |
| C8-C9 | 1.338(3) |
| C9-O3 | 1.360(2) |
| O3-C10 | 1.417(2) |
| C10-C11 | 1.321(3) |
| C10-C12 | 1.523(3) |
| C12-C8 | 1.507(3) |
| C7-O1 | 1.393(3) |
| C7-O2 | 1.211(3) |
| C1-Cl1 | 1.738(2) |
| Angles | |
| C8-C9-O3 | 114.13(18) |
| C9-O3-C10 | 106.39(16) |
| O3-C10-C11 | 118.8(2) |
| C11-C10-C12 | 131.1(2) |
| O3-C10-C12 | 110.07(16) |
| C10-C12-C8 | 99.46(17) |
| C12-C8-C9 | 109.77(18) |


3. Experimental
3.1. General
3.2. Synthesis of the 8-chloro-2-methylene-2,3-dihydrofuro[3,2-c]chromen-2-ones 4a–d
3.3. Synthesis of 8-chloro-3-(2-methoxyphenyl)-2-methyl-furo[3,2-c]chromen-2-one (5b)
3.4. X-ray Crystal Structure Determination of Compound 4a
| Empirical formula | C19H13O4Cl |
| Formula weight | 340.74 |
| Temperature | 150(2) K |
| Wavelength | 1.5418 Å |
| Crystal system, space group | triclinic, P-1 |
| Unit cell dimensions | a = 4.8366(2) Å α = 94.822(4)° |
| b = 11.0016(5) Å β = 90.363(4)° | |
| c = 14.6466(7) Å γ = 94.200(4)° | |
| Volume | 774.45(6) Å3 |
| Z, calculated density | 2, 1.461 mg/m3 |
| Absorption coefficient | 2.369 mm−1 |
| F(000) | 352 |
| Crystal size | 0.37 × 0.03 × 0.02 mm |
| Theta range for data collection | 3.03 to 73.76° |
| Limiting indices | −4 ≤ h ≤ 6, −13 ≤ k ≤ 12, −17 ≤ l ≤ 17 |
| Reflections collected / unique | 7403/2919 (Rint = 0.0214) |
| Completeness to theta = 73.76º | 93.4% |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restrains / parameters | 2919/0/269 |
| Goodness-of-fit on F2 | 1.166 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0411, wR2 = 0.1177 |
| R indices (all data) | R1 = 0.0517, wR2 = 0.1354 |
| Largest diff. peak and hole | 0.334 and −0.267 e∙Å3 |
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Sethna, S.M.; Shah, N.M. The chemistry of coumarins. Chem. Rev. 1945, 36, 1–62. [Google Scholar] [CrossRef]
- Murray, R.D.; Méndez, J.; Brown, S.A. The Natural Coumarins: Occurrence, Chemistry & Biochemistry; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Hoult, J.R.S.; Payá, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Coumarins: Biology, Applications and Mode of Action; O´Kennedy, R.; Thornes, R.D. (Eds.) John Wiley & Sons: Chichester, UK, 1997.
- Gambari, R.; Lampronti, I.; Bianchi, N.; Zuccato, C.; Viola, G.; Vedaldi, D.; Dall´Acqua, F. Structure and biological activity of furocoumarins. Top. Heterocycl. Chem. 2007, 9, 265–276. [Google Scholar]
- Conforti, F.; Marrelli, M.; Menichini, F.; Bonesi, M.; Statti, G.; Provenzano, E.; Menichini, F. Natural and synthetic furanocoumarins as treatment for vitiligo and psoriasis. Curr. Drug Ther. 2009, 4, 38–58. [Google Scholar] [CrossRef]
- Santana, L.; Uriarte, E.; Roleira, F.; Milhazes, N.; Borges, F. Furocoumarins in medicinal chemistry. Synthesis, applications, and biological activity. Curr. Med. Chem. 2004, 11, 3239–3261. [Google Scholar] [CrossRef]
- Traven, V.F. New synthetic routes to furocoumarins and their analogs: A review. Molecules 2004, 9, 50–66. [Google Scholar] [CrossRef]
- Lee, Y.R.; Byun, M.W.; Kim, B.S. Efficient one-step synthesis of 2-arylfurans by ceric ammonium nitrate (CAN)-mediated cycloaddition of 1,3-dicarbonyl compounds to alkynes. Bull. Korean Chem. Soc. 1998, 19, 1080–1083. [Google Scholar]
- Kobayashi, K.; Sakashita, K.; Akamatsu, H.; Tanaka, K.; Uchida, M.; Uneda, T.; Kitamura, T.; Morikawa, O.; Konishi, H. CAN-mediated formation of furopyranones and furoquinolinones. Heterocycles 1999, 51, 2881–2892. [Google Scholar] [CrossRef]
- Lee, Y.R.; Suk, J.Y.; Kim, B.S. Rhodium(II)-catalyzed reactions of 3-diazo-2,4-chromenediones. First one-step synthesis of pterophyllin 2. Tetrahedron Lett. 1999, 40, 6603–6607. [Google Scholar] [CrossRef]
- Tollari, S.; Palmisano, G.; Cenini, S.; Crovotto, G.; Giovenzana, G.B.; Penoni, A. Synthesis of furocoumarins via rhodium(II)-catalysed heterocyclisation of 3-diazobenzopyran-2,4(3H)-dione with terminal alkynes. Synthesis 2001, 735–740. [Google Scholar]
- Cheng, G.; Hu, Y. One-pot synthesis of furocoumarins through cascade addition-cyclization-oxidation. Chem. Commun. 2007, 3285–3287. [Google Scholar] [CrossRef]
- Cheng, G.; Hu, Y. Two efficient cascade reactions to synthesize substituted furocoumarins. J. Org. Chem. 2008, 73, 4732–4735. [Google Scholar] [CrossRef]
- Conreaux, D.; Belot, S.; Desbordes, P.; Monteiro, N.; Balme, G. Et3N-Induced demethylation-annulation of 3-alkynyl-4-methoxy-2-pyridonas and structurally related compounds in the synthesis of furan-fused heterocycles. J. Org. Chem. 2008, 73, 8619–8622. [Google Scholar]
- Raffa, G.; Rusch, M.; Balme, G.; Monteiro, N. A Pd-catalyzed heteroannulation approach to 2,3-disubstituted furo[3,2-c]coumarins. Org. Lett. 2009, 11, 5254–5257. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Xu, M.-H. One-pot synthesis of furocoumarins via sequential Pd/Cu-catalyzed alkynylation and intramolecular hydroalkoxylation. Org. Biomol. Chem. 2010, 8, 3073–3077. [Google Scholar] [CrossRef]
- Cadierno, V.; Gimeno, J.; Nebra, N. A novel propargylation/cycloisomerization tandem process catalyzed by a ruthenium(II)/trifluoroacetic acid system: One-pot entry to fully substituted furans from readily available secondary propargylic alcohols and 1,3-dicarbonyl compounds. Adv. Synth. Catal. 2007, 349, 382–394. [Google Scholar] [CrossRef]
- Albers, J.; Cadierno, V.; Crochet, P.; García-Garrido, S.E.; Gimeno, J. Octahedral ruthenium(II) complexes cis,cis-[RuX2(CNR)(CO)(P^P)] and cis,cis,cis-[RuX2(CO)2(P^P)] (X = Cl, Br; P^P = 1,1’-bis(diphenylphosphino)ferrocene, 1,1’-bis(diisopropylphosphino)ferrocene): Synthesis and catalytic applications in transfer hydrogenation of acetophenone and cycloisomerization of (Z)-3-methylpent-2-en-4-yn-1-ol. J. Organomet. Chem. 2007, 692, 5234–5244. [Google Scholar] [CrossRef]
- Cadierno, V.; Gimeno, J.; Nebra, N. One-pot three-component catalytic synthesis of fully substituted pyrroles from readily available propargylic alcohols, 1,3-dicarbonyl compounds and primary amines. Chem. Eur. J. 2007, 13, 9973–9981. [Google Scholar] [CrossRef]
- Cadierno, V.; Díez, J.; Gimeno, J.; Nebra, N. Ruthenium/TFA catalyzed coupling of activated secondary propargylic alcohols with cyclic 1,3-diones: Furan vs. pyran ring formation. J. Org. Chem. 2008, 73, 5852–5858. [Google Scholar] [CrossRef]
- Cadierno, V.; Crochet, P. Ruthenium-catalyzed furan- and pyrrole-ring formation. Curr. Org. Synth. 2008, 5, 343–364. [Google Scholar] [CrossRef]
- Cadierno, V.; Gimeno, J.; Nebra, N. One-pot three-component synthesis of tetrasubstituted N–H pyrroles from secondary propargylic alcohols, 1,3-dicarbonyl compounds and tert-butyl carbamate. J. Heterocycl. Chem. 2010, 47, 233–236. [Google Scholar]
- García-Garrido, S.E.; Francos, J.; Cadierno, V.; Basset, J.M.; Polshettiwar, V. Chemistry by nanocatalysis: First example of a solid-supported RAPTA complex for organic reactions in aqueous medium. ChemSusChem 2011, 4, 104–111. [Google Scholar] [CrossRef]
- Cadierno, V.; García-Garrido, S.E.; Gimeno, J.; Nebra, N. Atom-economic transformations of propargylic alcohols catalyzed by the 16-electron allyl-ruthenium(II) complex [Ru(η3-2-C3H4Me)(CO)(dppf)][SbF6] (dppf = 1,1’-bis(diphenylphosphino)ferrocene). Inorg. Chim. Acta 2010, 363, 1912–1934. [Google Scholar] [CrossRef]
- Huang, W.; Wang, J.; Shen, Q.; Zhou, X. Yb(OTf)3-catalyzed propargylation and allenylation of 1,3-dicarbonyl derivatives with propargylic alcohols: One-pot synthesis of multisubstituted furocoumarin. Tetrahedron 2007, 63, 11636–11643. [Google Scholar] [CrossRef]
- Microwaves in Organic Synthesis; Loupy, A. (Ed.) Wiley-VCH: Weinheim, Germany, 2006.
- Kappe, C.O.; Dallinger, D.; Murphee, S.S. Practical Microwave Synthesis for Organic Chemists; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Microwave Heating as a Tool for Sustainable Chemistry; Leadbeater, N.E. (Ed.) CRC Press: Boca Raton, FL, USA, 2011.
- Sawhney, K.N.; Mathur, K.B.L. Studies on some structural aspects of 4-hydroxycoumarin: Further extension of Meerwein´s diazo reaction and a substitution reaction involving 2-chloro-2-methylbut-yne. Indian J. Chem. B: Org. Chem. 1976, 14, 518–521. [Google Scholar]
- Chênevert, R.; Pagé, J.; Plante, R.; Beaucage, D. Synthesis of 4,4-dimethyl-5-methylene-4,5-dihydrofurans. Synthesis 1982, 75–77. [Google Scholar]
- Chênevert, R.; Pagé, J.; Voyer, N. Synthesis of (±)-dehydroxyglaupalol and analogs. Synth. Commun. 1984, 14, 737–742. [Google Scholar] [CrossRef]
- Reisch, J.; Dharmaratne, H.R.W. A convenient synthesis of the 2-dimethyl-2H-chromene system. Z. Naturforsch. B 1985, 40, 636–638. [Google Scholar]
- Mitra, J.; Mitra, A.K. Palladium(II) assisted cyclization of hydroxyallylcoumarins. Indian J. Chem. B: Org. Chem. 1994, 33, 276–279. [Google Scholar]
- Berger, S.; Haak, E. Ruthenium-catalyzed addition of carboxylic acids or cyclic 1,3-dicarbonyl compounds to propargylic alcohols. Tetrahedron Lett. 2010, 51, 6630–6634. [Google Scholar] [CrossRef]
- Cadierno, V.; Díez, J.; García-Garrido, S.E.; Gimeno, J. Ru(η3-2-C3H4Me)(CO)(dppf)][SbF6]: A mononuclear 16e− ruthenium(II) catalyst for propargylic substitution and isomerization of HCCCPh2(OH). Chem. Commun. 2004, 2716–2717. [Google Scholar]
- Midland, M.M. Preparation of monolithium acetylide in tetrahydrofuran. Reaction with aldehydes and ketones. J. Org. Chem. 1975, 40, 2250–2252. [Google Scholar] [CrossRef]
- CrysAlisPro CCD & CrysAlisPro RED; Oxford Diffraction Ltd.: Oxford, UK, 2008.
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL97: Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- International Tables for X-Ray Crystallography; Kynoch Press: Birminghan, UK, 1974; Volume IV, (present distributor: Kluwer Academic Publishers: Dordrecht, The Netherlands).
- Spek, A.L. PLATON: A multipurpose Crystallographic Tool; University of Utrecht: Utrecht, The Netherlands, 2006. [Google Scholar]
- Sample Availability: Samples of the compounds 4a–d and 5b are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nebra, N.; Díaz-Álvarez, A.E.; Díez, J.; Cadierno, V. Expeditious Entry to Novel 2-Methylene-2,3-dihydrofuro[3,2-c] chromen-2-ones from 6-Chloro-4-hydroxychromen-2-one and Propargylic Alcohols. Molecules 2011, 16, 6470-6480. https://doi.org/10.3390/molecules16086470
Nebra N, Díaz-Álvarez AE, Díez J, Cadierno V. Expeditious Entry to Novel 2-Methylene-2,3-dihydrofuro[3,2-c] chromen-2-ones from 6-Chloro-4-hydroxychromen-2-one and Propargylic Alcohols. Molecules. 2011; 16(8):6470-6480. https://doi.org/10.3390/molecules16086470
Chicago/Turabian StyleNebra, Noel, Alba E. Díaz-Álvarez, Josefina Díez, and Victorio Cadierno. 2011. "Expeditious Entry to Novel 2-Methylene-2,3-dihydrofuro[3,2-c] chromen-2-ones from 6-Chloro-4-hydroxychromen-2-one and Propargylic Alcohols" Molecules 16, no. 8: 6470-6480. https://doi.org/10.3390/molecules16086470