1,1’-(3-Methyl-4-phenylthieno[2,3-b]thiophene-2,5-diyl)diethanone as a Building Block in Heterocyclic Synthesis. Novel Synthesis of Some Pyrazole and Pyrimidine Derivatives
Abstract
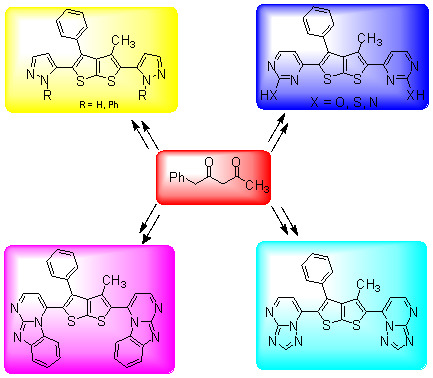
:1. Introduction
2. Results and Discussion






3. Experimental
3.1. General
3.2. 1,1'-(3-Methyl-4-phenylthieno[2,3-b]thiophene-2,5-diyl)diethanone (2)
3.3. 1,1'-(3-Methyl-4-phenylthieno[2,3-b]thiophene-2,5-diyl)bis(3-(dimethylamino)prop-2-en-1-one) (3)
3.4. General Procedure for the Synthesis of Compounds 4a–c (GP1)
3.5. General Procedure for the Synthesis of Compounds 5a–b (GP2)
3.6. General Procedure for the Synthesis of Compounds 6,7 (GP3)
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Mabkhot, Y.N.; Kheder, N.A.; Al-Majid, A.M. Facile and convenient synthesis of new thieno[2,3-b]thiophene derivatives. Molecules 2010, 15, 9418–9426. [Google Scholar] [CrossRef]
- Mabkhot, Y.N. Synthesis and analysis of some bis-heterocyclic compounds containing sulphur. Molecules 2009, 14, 1904–1914. [Google Scholar] [CrossRef]
- Boydston, A.J.; Vu, P.D.; Dykhno, O.L.; Chang, V.; Wyatt, A.R.; Stockett, A.S.; Ritschdorff, E.T.; Shear, J.B.; Bielawski, C.W. Modular fluorescent benzobis(imidazolium) salts: Syntheses, photophysical analyses, and applications. J. Am. Chem. Soc. 2008, 130, 3143–3156. [Google Scholar]
- Coady, D.J.; Khramov, D.M.; Norris, B.C.; Tennyson, A.G.; Bielawski, C.W. Adapting N-heterocyclic carbene/azide coupling chemistry for polymer synthesis: Enabling access to aromatic polytriazenes. Angew. Chem. Int. Ed. 2009, 48, 5187–5190. [Google Scholar] [CrossRef]
- Tang, T.; Coady, D.J.; Boydston, A.J.; Dykhno, O.L.; Bielawski, C.W. Pro-ionomers: An anion metathesis approach to amphiphilic block ionomers from neutral precursors. Adv. Mater. 2008, 20, 3096–3099. [Google Scholar] [CrossRef]
- King, W.J.; Nord, F.F. Studies in the thiophene series. V. Wolff-Kishner reductions. J. Org. Chem. 1949, 14, 638–642. [Google Scholar] [CrossRef]
- Wu, C.; Decker, E.R.; Blok, N.; Bui, H.; You, T.J.; Wang, J.; Bourgoyne, A.R.; Knowles, V.; Berens, K.L.; Holland, G.W.; et al. Discovery, modeling, and human pharmacokinetics of N-(2-acetyl-4,6-dimethylphenyl)-3-(3,4-dimethylisoxazol-5-ylsulfamoyl)-thiophene-2-carboxamide (TBC3711), a second generation, ETA selective, and orally bioavailable endothelin antagonist. J. Med. Chem. 2004, 47, 1969–1986. [Google Scholar] [CrossRef]
- Dubus, S.; Levesque, I.; Brunette, M.; Corbeil, G.; Boissinot, M.; Boivin, G.; Bergeron, M.G.; Boudreau, D.; Leclerc, M. Fluorescent polymeric transducer for the rapid, simple, and specific detection of nucleic acids at the zeptomole level. J. Am. Chem. Soc. 2004, 126, 4240–4244. [Google Scholar]
- Rost, C.; Karg, S.; Riess, W.; Loi, M.A.; Murgia, M.; Muccini, M. Ambipolar light-emitting organic field-effect transistor. Appl. Phys. Lett. 2004, 85, 1613–1615. [Google Scholar] [CrossRef]
- Vriezema, D.M.; Hoogboom, J.; Velonia, K.; Takazawa, K.; Christianen, P.C.M.; Maan, J.C.; Rowan, A.E.; Nolte, R.J. Vesicles and polymerized vesicles from thiophene-containing rod-coil block copolymers. Angew. Chem. Int. Ed. 2003, 42, 772–776. [Google Scholar] [CrossRef]
- Pullen, A.E.; Bushel, M.G.; Swager, T.M. Charge-specific interactions in segmented conducting polymers: An approach to selective ionoresistive responses. Angew. Chem. Int. Ed. 2004, 43, 3700–3703. [Google Scholar] [CrossRef]
- Heeney, M.; Bailey, C.; Genevicius, K.; Shkunov, M.; Sparrowe, D.; Tierney, S.; Mculloch, I. Stable polythiophene semiconductors incorporating thieno[2,3-b]thiophene. J. Am. Chem. Soc. 2005, 127, 1078–1079. [Google Scholar]
- Mashraqui, S.H.; Sangvikar, Y.S.; Meetsma, A. Synthesis and structures of thieno[2,3-b]thiophene incorporated [3.3]dithiacyclophanes. Enhanced first hyperpolarizability in an unsymmetrically polarized cyclophane. Tetrahedron Lett. 2006, 47, 5599–5602. [Google Scholar] [CrossRef]
- Mashraqui, S.H.; Sangvikar, Y.S.; Ashraf, M.; Kumar, S.; Daub, E. Dipyridyl/pyridinium thieno[2,3-b]thiophenes as new atropisomeric systems. Synthesis, conformat-ional analysis and energy minimization. Tetrahedron 2005, 61, 3507–3513. [Google Scholar] [CrossRef]
- Leriche, P.R.J.; Turbiez, M.M.; Monroche, V.; Allain, M.; Sauvage, F.X.; Roncali, J.; Frere, P.; Skabara, P.J. Linearly extended tetrathiafulvalene analogues with fused thiophene units as π conjugated spacers. J. Mater. Chem. 2003, 13, 1324–1327. [Google Scholar] [CrossRef]
- Lee, B.; Seshadri, V.; Palko, H.; Sotzing, G.A. Ring-sulfonated poly(thienothiophene). J. Adv. Mater. 2005, 17, 1792–1795. [Google Scholar] [CrossRef]
- Lim, E.; Jung, B.J.; Lee, J.; Shim, H.K.; Lee, J.I.; Yang, Y.S.; Do, L.M. Thin-film morphologies and solution-processable field-effect transistor behavior of a fluorine-thieno[3,2-b]thiophene-based conjugated copolymer. Macromolecules 2005, 38, 4531–4535. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.H.; Kim, T.H.; Noh, Y.Y.; Pyo, S.; Yi, M.H.; Kim, D.Y.; Kwon, S.K. Synthesis and studies on 2-hexylthieno[3,2-b]thiophene end-capped oligomers for OTFTs. Chem. Mater. 2007, 19, 3561–3567. [Google Scholar] [CrossRef]
- Jarak, I.; Kralj, M.; Piantanida, I.; Suman, L.; Zinic, M.; Pavelic, K.; Karminski-Zamola, G. Novel cyano- and amidino-substituted derivatives of thieno[2,3-b]- and thien- o[3,2-b]thiophene-2-carboxanilides and thieno[30,20:4,5]thieno- and thieno [20,30:4,5] thieno[2,3-c]quinolones: Synthesis, photochemical synthesis, DNA binding, and antitumor evaluation. Bioorg. Med. Chem. 2006, 14, 2859–2868. [Google Scholar] [CrossRef]
- Peters, D.; Hornfeldt, A.B.; Gronowitz, S. Synthesis of various 5-substituted uracils. J. Heterocycl. Chem. 1990, 27, 2165–2173. [Google Scholar] [CrossRef]
- Kukolja, S.; Draheim, S.E.; Graves, B.J.; Hunden, D.C.; Pfeil, J.L.; Cooper, R.D.G.; Ott, J.L.; Couter, F.T. Orally absorbable cephalosporin antibiotics. 2. Structure-activity studies of bicyclic glycine derivatives of 7-aminodeacetoxycephalosporanic acid. J. Med. Chem. 1985, 28, 1896–1903. [Google Scholar] [CrossRef]
- Prugh, J.D.; Hartman, G.D.; Mallorga, P.J.; McKeever, B.M.; Michelson, S.R.; Murcko, M.A.; Schwam, H.; Smith, R.L.; Sondey, J.M.; Springer, J.P.; et al. New isomeric classes of topically active ocular hypotensive carbonic anhydrase inhibitors: 5-substituted thieno[2,3-b]thiophene-2-sulfonamides and 5-substituted thieno[3,2-b]thiophene-2-sulfonamides. J. Med. Chem. 1991, 34, 1805–1818. [Google Scholar] [CrossRef]
- Egbertson, M.S.; Cook, J.J.; Bednar, B.; Prugh, J.D.; Bednar, R.A.; Gaul, S.L.; Gould, R.J.; Hartman, G.D.; Homnick, C.F.; Holahan, M.A.; et al. Centrally constrained thienothiophene α-sulfonamides are potent, long acting in vivo inhibitors of platelet aggregation. J. Med. Chem. 1999, 42, 2409–2421. [Google Scholar] [CrossRef]
- Allard, S.; Forster, M.; Souharce, B.; Thiem, H.; Scherf, U. Organic semiconductors for solution-processable field-effect transistors (OFETs). Angew. Chem. Int. Ed. 2008, 47, 4070–4098. [Google Scholar] [CrossRef]
- Wu, Z.-Q.; Ono, R.J.; Chen, Z.; Bielawski, C.W. Synthesis of poly(3-alkylthiophene)-block-poly(arylisocyanide): Two sequential, mechanistically distinct polymerizations using a single catalyst. J. Am. Chem. Soc. 2010, 132, 14000–14001. [Google Scholar] [CrossRef]
- Li, Z.; Ono, R.J.; Wu, Z.-Q.; Bielawski, C.W. Synthesis and self-assembly of poly(3-hexylthiophene)-block-poly(acrylic acid). Chem. Commun. 2011, 47, 197–199. [Google Scholar]
- Wu, Z.-Q.; Ono, R.J.; Chen, Z.; Li, Z.; Bielawski, C.W. Polythiophene-block-poly(γ-benzyl L-glutamate): Synthesis and study of a new rod-rod block copolymer. Polym. Chem. 2011, 2, 300–302. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Brown, P.J.; Friend, R.H.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.M.W.; Spiering, A.J.H.; Janssen, R.A.J.; Meijer, E.W.; Herwig, P.; et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999, 401, 685–688. [Google Scholar] [CrossRef]
- Schuett, W.; Raoort, H.S. The paralytic shellfish poison. Degradation to a pyrrolopyrimidine. J. Am. Chem. Soc. 1962, 84, 2266–2267. [Google Scholar] [CrossRef]
- Ho, Y.W. 5-(1-Pyrrolyl)-2-phenylthieno[2,3-d]pyrimidine as building block in heterocyclic synthesis: Novel synthesis of some pyrazoles, pyrimidines, imidazo[1,2-a]pyrimidines, pyrazolo[1,5-a]pyrimidines, pyrido-(pyrimido)pyrazolo[1,5-a]pyrimidines, 1,2,4-triazolo[1,5-a]pyrimidine and a 1,2,3,4-Tetrazolo[1,5-a]pyrimidine derivative. J. Chin. Chem. Soc. 2007, 54, 1075–1085. [Google Scholar]
- Alexander, S.K.; Smith, L. Novel one pot synthesis of polysubstituted pyrazolo[1,5-a] and imidazo[1,2-a]pyrimidines. Tetrahedron Lett. 2006, 47, 2611–2614. [Google Scholar] [CrossRef]
- Riyadh, S.M. Enaminones as building blocks for the synthesis of substituted pyrazoles with antitumor and antimicrobial activities. Molecules 2011, 16, 1834–1853. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2-7 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mabkhot, Y.N.; Al-Majid, A.M.; Barakat, A.; Alshahrani, S.; Siddiqui, Y. 1,1’-(3-Methyl-4-phenylthieno[2,3-b]thiophene-2,5-diyl)diethanone as a Building Block in Heterocyclic Synthesis. Novel Synthesis of Some Pyrazole and Pyrimidine Derivatives. Molecules 2011, 16, 6502-6511. https://doi.org/10.3390/molecules16086502
Mabkhot YN, Al-Majid AM, Barakat A, Alshahrani S, Siddiqui Y. 1,1’-(3-Methyl-4-phenylthieno[2,3-b]thiophene-2,5-diyl)diethanone as a Building Block in Heterocyclic Synthesis. Novel Synthesis of Some Pyrazole and Pyrimidine Derivatives. Molecules. 2011; 16(8):6502-6511. https://doi.org/10.3390/molecules16086502
Chicago/Turabian StyleMabkhot, Yahia Nasser, Abdullah Mohammed Al-Majid, Assem Barakat, Saeed Alshahrani, and Yamin Siddiqui. 2011. "1,1’-(3-Methyl-4-phenylthieno[2,3-b]thiophene-2,5-diyl)diethanone as a Building Block in Heterocyclic Synthesis. Novel Synthesis of Some Pyrazole and Pyrimidine Derivatives" Molecules 16, no. 8: 6502-6511. https://doi.org/10.3390/molecules16086502