Analgesic and Anti-Inflammatory Activities of Salicylaldehyde 2-Chlorobenzoyl Hydrazone (H2LASSBio-466), Salicylaldehyde 4-Chlorobenzoyl Hydrazone (H2LASSBio-1064) and Their Zinc(II) Complexes
Abstract
:1. Introduction

2. Results and Discussion
2.1. Formation of the Zinc(II) Complexes 1 and 2

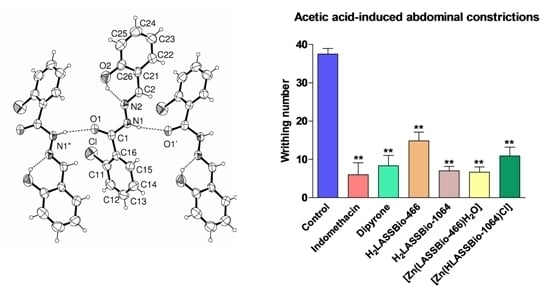
2.2. Crystal Structure of H2LASSBio-466
| Compound | H2LASSBio-466 | |
|---|---|---|
| Empirical Formula | C14H11ClN2O2 | |
| Formula Weight | 274.70 | |
| Temperature, K | 294(2) | |
| Crystal System | Monoclinic | |
| Space Group | P21/c | |
| Unit cell dimensions | a, Å | 9.889(1) |
| b, Å | 13.360(2) | |
| c, Å | 10.079(1) | |
| α, ° | 90 | |
| β, ° | 93.10(1) | |
| γ, ° | 90 | |
| Volume, Å3 | 1329.7(3) | |
| Z, Density calc., Mg/m3 | 4, 1.372 | |
| Absorption coefficient, mm−1 | 0.286 | |
| F(000) | 568 | |
| Crystal size, mm | 0.20 × 0.12 × 0.08 | |
| Crystal color / shape | Colorless / prism | |
| θ range for data coll. | 3.05 to 24.08º | |
| Index range | −10 ≤ h ≤11 | |
| −15 ≤ k ≤ 15 | ||
| −11 ≤ l ≤ 11 | ||
| Completeness, θ = 26.37° | 99.6 % | |
| Max. / min. transmission | 0.977 / 0.945 | |
| Goodness–of–fit on F2 | 1.016 | |
| Reflec. collect./unique (Rint) | 5646/2095 (0.032) | |
| Observed reflections [I > 2σ(I)] | 1612 | |
| Weights, w | [σ2(Fo2) + (0.088P)2 + 0.22P]-1 P = [Max((Fo2,0) + 2Fc2]/3 | |
| Data / restraints / parameters | 2095 / 0 / 184 | |
| Final R indexesa [I > 2σ(I)] | R1 = 0.0474, wR2 = 0.1296 | |
| R indices (all data) | R1 = 0.0637, wR2 = 0.1479 | |
| Extinction coefficient | 0.07(1) | |
| Larg. peak & hole, e Å−3 | 0.180 / -0.246 | |
| Atoms | Bond lengths (Å) | Atoms | Angles (°) |
|---|---|---|---|
| Cl-C(10) | 1.724(3) | O(2)-C(8)-C(9) | 123.2(2) |
| O(1)-C(1) | 1.353(3) | N(1)-C(7)-C(2) | 121.1(2) |
| O(2)-C(8) | 1.226(3) | N(2)-C(8)-C(9) | 114.7(2) |
| N(1)-N(2) | 1.376(3) | N(1)-N(2)-C(8) | 118.6(2) |
| N(1)-C(7) | 1.272(3) | N(2)-N(1)-C(7) | 117.2(2) |
| N(2)-C(8) | 1.346(3) | C(1)-C(2)-C(3) | 118.3(2) |
| C(1)-C(2) | 1.400(3) | C(1)-C(2)-C(7) | 122.2(2) |
| C(1)-C(6) | 1.384(4) | C(1)-C(6)-C(5) | 120.5(3) |
| C(2)-C(3) | 1.390(3) | C(2)-C(1)-C(6) | 119.7(2) |
| C(2)-C(7) | 1.445(3) | C(2)-C(3)-C(4) | 121.1(3) |
| C(3)-C(4) | 1.374(4) | C(3)-C(4)-C(5) | 119.5(3) |
| C(4)-C(5) | 1.371(5) | C(3)-C(2)-C(7) | 119.5(2) |
| C(5)-C(6) | 1.359(4) | C(4)-C(5)-C(6) | 120.8(3) |
| C(8)-C(9) | 1.494(3) | C(8)-C(9)-C(10) | 122.4(2) |
| C(9)-C(10) | 1.393(3) | C(8)-C(9)-C(14) | 119.9(2) |
| C(9)-C(14) | 1.388(3) | C(9)-C(10)-Cl | 120.8(2) |
| C(10)-C(11) | 1.381(4) | C(9)-C(10)-C(11) | 121.1(3) |
| C(11)-C(12) | 1.376(4) | C(9)-C(14)-C(13) | 121.5(3) |
| C(12)-C(13) | 1.371(4) | C(10)-C(9)-C(14) | 117.6(2) |
| C(13)-C(14) | 1.370(3) | C(10)-C(11)-C(12) | 119.5(3) |
| C(11)-C(10)-Cl | 118.1(2) | ||
| C(11)-C(12)-C(13) | 120.3(3) | ||
| C(12)-C(13)-C(14) | 119.9(3) | ||
| O(1)-C(1)-C(2) | 122.4(2) | ||
| O(1)-C(1)-C(6) | 118.0(2) | ||
| O(2)-C(8)-N(2) | 122.1(2) |
2.3. Spectroscopic Characterization
2.4. Anti-Nociceptive Activity of H2LASSBio-466, H2LASSBio-1064, [Zn(LASSBio-466)H2O]2 (1) and [Zn(HLASSBio-1064)Cl]2 (2)
| Substance | n | Writhing number Mean ± S.E.M. | % of inhibition Mean ± S.E.M. |
|---|---|---|---|
| Control | 6 | 37.5 ± 1.4 | ___ |
| Indomethacin | 6 | 6.0 ± 3.1 ** | 84.0 ± 8.3 ** |
| Dipyrone | 6 | 8.3 2.7 ** | 77.8 ± 7.2 ** |
| H2LASSBio-466 | 6 | 14.8 ± 2.2 ** | 60.4 ± 6.0 ** |
| H2LASSBio-1064 | 6 | 7.0 ± 1.1 ** | 81.3 ± 3.0 ** |
| [Zn(LASSBio-466)H2O]2 (1) | 6 | 6.6 ± 1.4 ** | 82.3 ± 3.7 ** |
| [Zn(HLASSBio-1064)Cl]2 (2) | 6 | 10.8 ± 2.3 ** | 70.9 ± 6.2 ** |
| Substance | n | Phase 1 Mean ± S.E.M. | Phase 2 Mean ± S.E.M. | % of inhibition (Mean ± S.E.M.) | |
|---|---|---|---|---|---|
| Phase 1 | Phase 2 | ||||
| Control | 5 | 54.8 ± 2,3 | 227.6 ± 22.7 | — | — |
| Indomethacin | 5 | 57.1 ± 8.5 | 115.9 ± 3.3 * | 0 | 49.1 ± 1.4 * |
| H2LASSBio-466 | 5 | 29.3 ± 8.3 ** | 182.4 ± 17.4 | 46.5 ± 8.3 ** | 19.8 ± 9.7 |
| H2LASSBio-1064 | 5 | 25.7 ± 4.9 * | 117.0 ± 19.1 * | 53.1 ± 8.9 * | 48.5 ± 8.4 * |
| [Zn(LASSBio-466)H2O]2 (1) | 5 | 40.6 ± 12.7 | 142.8 ± 23.1 * | 25.9 ± 15.7 | 37.3 ± 10.1 * |
| [Zn(HLASSBio-1064)Cl]2 (2) | 5 | 39.6 ± 7.8 | 161.3 ± 36.0 | 27.7 ± 13.1 | 29.2 ± 13.9 |
| Substance | n | Mean ± S.E.M. | ||||
|---|---|---|---|---|---|---|
| 0 min | 60 min | 90 min | 120 min | 150 min | ||
| Control | 6 | 2.8 ± 0.2 | 2.9 ± 0.3 | 1.6 ± 0.2 | 2.3 ± 0.40 | 1.7 ± 0.1 |
| Morphine | 6 | 1.8 ± 0.5 | 9.0 ± 1.6 * | 7.4 ± 0.8 * | 5.3 ± 0.8 * | 2.5 ± 0.2 |
| H2LASSBio-466 | 6 | 2.1 ± 0.21 | 2.3 ± 0.4 | 2.1 ± 0.2 | 2.2 ± 0.2 | 2.6 ± 0.1 |
| H2LASSBio-1064 | 6 | 1.7 ± 0.18 | 2.6 ± 0.2 | 2.1 ± 0.2 | 2.5 ± 0.3 | 3.5 ± 0.3 |
| [Zn(LASSBio-466)H2O]2 (1) | 6 | 2.1 ± 0.2 | 4.2 ± 0.8 | 2.4 ± 0.6 | 3.6 ± 0.6 | 5.4 ± 1.2 |
| [Zn(HLASSBio-1064)Cl]2 (2) | 6 | 1.5 ± 0.1 | 2.0 ± 0.5 | 2.3 ± 0.5 | 2.9 ± 1.0 | 1.9 ± 0.2 |
| Substance | n | Cell Number × 106/mL Mean ± S.E.M. | % of inhibition Mean ± S.E.M. |
|---|---|---|---|
| Control | 7 | 38.0 ± 1.0 | ___ |
| Saline | 7 | 5.0 ± 0.8 | ___ |
| Indomethacin | 7 | 17.7± 1.0 ** | 53.4 ± 2.7 ** |
| H2LASSBio-466 | 7 | 11.4 ± 1.4 ** | 70.0 ± 3.8 ** |
| H2LASSBio-1064 | 7 | 8.4 ± 0.9 ** | 77.8 ± 2.4 ** |
| [Zn(LASSBio-466)H2O]2 (1) | 7 | 10.7 ± 1.8 ** | 71.8 ± 4.9 ** |
| [Zn(HLASSBio-1064)Cl]2 (2) | 7 | 13.4 ± 1.5 ** | 64.7 ± 4.0 ** |
3. Experimental
3.1. General
3.2. Synthesis of H2LASSBio-466 and H2LASSBio-1064
3.3. Synthesis of Zinc(II) Complexes
3.3.1. Synthesis of [Zn(LASSBio-466)H2O]2 (1) and [Zn(HLASSBio-1064)Cl]2 (2)
3.4. X-ray Crystallography
3.5. Anti-nociceptive and anti-inflammatory activities of H2LASSBio-466, H2LASSBio-1064, [Zn(LassBio-466)H2O]2 (1) and [Zn(HLassBio-1064)Cl]2 (2)
3.5.1. Animals
3.5.2. Writhing Test
3.5.3. Formalin-Induced Pain in Mice
3.5.4. Hot-Plate Test
3.5.5. Zymosan-Induced Peritonitis
3.5.6. Statistical Analysis
4. Conclusions
Acknowledgements
Supplementary Material
References
- Yong, V.W. Inflammation in neurological disorders: A help or a hindrance? Neuroscientist 2010, 16, 408–420. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Campbell, E.L.; Colgan, S.P. Metabolic shifts in immunity and inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Szewczyk, B.; Kubera, M.; Nowak, G. The role of zinc in neurodegenerative inflammatory pathways in depression. Prog. Neuro-Psycopharmacol. Biol. Psychiatry 2011, 35, 693–701. [Google Scholar] [CrossRef]
- Zalewski, P.D.; Truong-Tran, A.Q.; Grosser, D.; Jayaram, L.; Murgia, C.; Ruffin, R.E. Zinc metabolism in airway epithelium and airway inflammtion: basic mechanism and clinical targets. A review. Pharmacol. Ther. 2005, 105, 127–149. [Google Scholar] [CrossRef]
- Parrilha, G.L.; Vieira, R.P.; Rebolledo, A.P.; Mendes, I.C.; Lima, L.M.; Barreiro, E.J.; Piro, O.E.; Castellano, E.E.; Beraldo, H. Binuclear zinc(II) complexes with the anti-inflammatory compounds salicylaldehyde semicarbazone and salicylaldehyde-4-chlorobenzoyl hydrazone. Polyhedron 2011, 30, 1891–1898, and references therein.. [Google Scholar] [CrossRef]
- Johnson, C.K. ORTEP; Oak Ridge National Laboratory: Tennessee, 1965; Report ORNL-3794. [Google Scholar]
- Salavati-Niasari, M.; Amiri, A. Synthesis and characterization of alumina-supported Mn(II), Co(II), Ni(II) and Cu(II) complexes of bis(salicylaldiminato)hydrazone as catalysts for oxidation of cyclohexene with tert-buthylhydroperoxide. Appl. Catal. A Gen. 2005, 290, 46–53. [Google Scholar] [CrossRef]
- Despaigne, A.A.R.; Da Silva, J.G.; Do Carmo, A.C.M.; Sives, F.; Piro, O.E.; Castellano, E.E.; Beraldo, H. Copper(II) and zinc(II) complexes with 2-formylpyridine-derived hydrazones. Polyhedron 2009, 28, 3797–3803. [Google Scholar] [CrossRef]
- Despaigne, A.A.R.; Vieira, L.F.; Mendes, I.C.; Da Costa, F.B.; Speziali, N.L.; Beraldo, H. Organotin(IV) complexes with 2-acetylpyridine benzoyl hydrazones: Antimicrobial activity. J. Braz. Chem. Soc. 2010, 21, 1247–1257. [Google Scholar] [CrossRef]
- Despaigne, A.A.R.; Da Silva, J.G.; Do Carmo, A.C.M.; Piro, O.E.; Castellano, E.E.; Beraldo, H. Structural studies on zinc(II) complexes with 2-benzoylpyridine-derived hydrazones. Inorg. Chim. Acta 2009, 362, 2117–2122. [Google Scholar] [CrossRef]
- Despaigne, A.A.R.; Da Silva, J.G.; Do Carmo, A.C.M.; Piro, O.E.; Castellano, E.E.; Beraldo, H. Copper(II) and zinc(II) complexes with 2-benzoylpyridine-methyl hydrazone. J. Mol. Struct. 2009, 920, 97–102. [Google Scholar] [CrossRef]
- Coolier, H.O.J.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. Abdominal constriction response and its suppression by analgesic drugs in the mouse. Brit. J. Pharmacol. 1968, 32, 295–310. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and noninflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics. II. Dithienylbutenylamines and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953, 107, 385–393. [Google Scholar]
- Doherty, N.S.; Poubelle, P.; Borgeat, P.; Beaver, T.H.; Westrich, G.L.; Schrader, N.L. Intraperitoneal injection of zymosan in mice induces pain, inflammation and the synthesis of peptidoleukotrienes and prostaglandin E2. Prostaglandins 1985, 30, 769–789. [Google Scholar] [CrossRef]
- Da Silva, Y.K.C.; Augusto, C.V.; Barbosa, M.L.C.; Melo, G.M.A.; De Queiroz, A.C.; Dias, T.L.M.F.; Bispo, W., Jr.; Barreiro, E.J.; Lima, L.M.; Alexandre-Moreira, M.S. Synthesis and pharmacological evaluation of pyrazine N-acylhydrazone derivatives designed as novel analgesic and anti-inflammatory drug candidates. Bioorg. Med. Chem. 2010, 18, 5007–5015. [Google Scholar]
- Dutta, M.M.; Goswami, B.N.; Kataky, J.C.S. Studies on biologically-active heterocycles. III. Synthesis and antibacterial activity of some 2-aryl/arakyl-3-substituted-4-thiazoldinones. J. Indian Chem. Soc. 1990, 67, 332–334. [Google Scholar]
- Ainscough, E.W.; Brodie, A.M.; Denny, W.A.; Finlay, G.J.; Gothe, S.A.; Ranford, J.D. Cytotoxicity of salicylaldehyde benzoylhydrazone analogs and their transition metal complexes: quantitative structure-activity relationships. J. Inorg. Biochem. 1999, 77, 125–133. [Google Scholar] [CrossRef]
- Enraf-Nonius, COLLECT; Nonius BV: Delft, The Netherlands, 1997-2000.
- Otwinowski, Z.; Minor, W. Methods in Enzymology; Carter, C.W. Jr., Sweet, R.M., Eds.; Academic Press: New York, NY, USA, 1997; pp. 307–326. [Google Scholar]
- Sheldrick, G.M. SHELXS-97. Program for Crystal Structure Resolution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97. Program for Crystal Structures Analysis; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sample Availability: Samples of the compounds H2LASSBio-466, H2LASSBio-1064 and complexes [Zn(LASSBio-466)H2O]2 (1) and [Zn(HLASSBio-1064)Cl]2 (2) are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Júnior, W.B.; Alexandre-Moreira, M.S.; Alves, M.A.; Perez-Rebolledo, A.; Parrilha, G.L.; Castellano, E.E.; Piro, O.E.; Barreiro, E.J.; Lima, L.M.; Beraldo, H. Analgesic and Anti-Inflammatory Activities of Salicylaldehyde 2-Chlorobenzoyl Hydrazone (H2LASSBio-466), Salicylaldehyde 4-Chlorobenzoyl Hydrazone (H2LASSBio-1064) and Their Zinc(II) Complexes. Molecules 2011, 16, 6902-6915. https://doi.org/10.3390/molecules16086902
Júnior WB, Alexandre-Moreira MS, Alves MA, Perez-Rebolledo A, Parrilha GL, Castellano EE, Piro OE, Barreiro EJ, Lima LM, Beraldo H. Analgesic and Anti-Inflammatory Activities of Salicylaldehyde 2-Chlorobenzoyl Hydrazone (H2LASSBio-466), Salicylaldehyde 4-Chlorobenzoyl Hydrazone (H2LASSBio-1064) and Their Zinc(II) Complexes. Molecules. 2011; 16(8):6902-6915. https://doi.org/10.3390/molecules16086902
Chicago/Turabian StyleJúnior, Walfrido Bispo, Magna S. Alexandre-Moreira, Marina A. Alves, Anayive Perez-Rebolledo, Gabrieli L. Parrilha, Eduardo E. Castellano, Oscar E. Piro, Eliezer J. Barreiro, Lídia Moreira Lima, and Heloisa Beraldo. 2011. "Analgesic and Anti-Inflammatory Activities of Salicylaldehyde 2-Chlorobenzoyl Hydrazone (H2LASSBio-466), Salicylaldehyde 4-Chlorobenzoyl Hydrazone (H2LASSBio-1064) and Their Zinc(II) Complexes" Molecules 16, no. 8: 6902-6915. https://doi.org/10.3390/molecules16086902
APA StyleJúnior, W. B., Alexandre-Moreira, M. S., Alves, M. A., Perez-Rebolledo, A., Parrilha, G. L., Castellano, E. E., Piro, O. E., Barreiro, E. J., Lima, L. M., & Beraldo, H. (2011). Analgesic and Anti-Inflammatory Activities of Salicylaldehyde 2-Chlorobenzoyl Hydrazone (H2LASSBio-466), Salicylaldehyde 4-Chlorobenzoyl Hydrazone (H2LASSBio-1064) and Their Zinc(II) Complexes. Molecules, 16(8), 6902-6915. https://doi.org/10.3390/molecules16086902