Use of Graphite Oxide and Graphene Oxide as Catalysts in the Synthesis of Dipyrromethane and Calix[4]pyrrole
Abstract
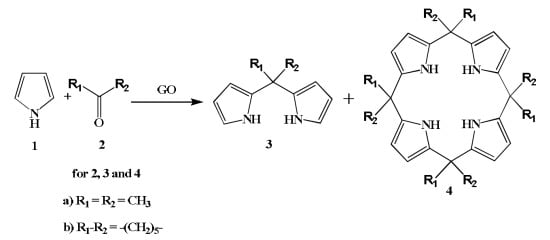
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. Preparation of Authentic Samples
3.3. Preparation of Catalysts
3.3.1. Graphite Oxide
3.3.2. Graphene Oxide
3.3.3. Preparation of Reduced Graphene Oxide
3.4. The Reaction of Pyrrole and Acetone in Organic Solvents
3.5. The Reaction of Pyrrole and Acetone in Aqueous Solution
3.6. The Reaction of Pyrrole and Acetone in Aqueous Solution in Presence of Suphonate Salts
3.7. The Reaction of Pyrrole and Acetone Catalyzed by Reduced Graphene Oxide under Different Conditions
4. Conclusions
Acknowledgements
References
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis, molecular engineering, thinfilm, hybrids and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ratianc, K.R.; Ringer, S.P.; Thordason, P.; Gooding, J.J.; Braet, F. Carbon naonmaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.K.; Choi, M.-C.; Kong, J.-Y.; Kim, G.Y.; Kim, M.J.; Kim, S.-H.; Mishra, S.; Singh, R.P.; Ha, C.-S. Synthesis and drug delivery behaviour of chitosan-functionalized graphene oxide hybrid nanosheets. Macromol. Mater. Eng. 2011, 296, 131–140. [Google Scholar] [CrossRef]
- Kamat, P.V. Graphene based nanoassemblies for energy conversion. J. Phys. Chem. Lett. 2011, 2, 242–251. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morojov, S.V.; Jiang, D.; Zhang, Y.; Dobonos, S.V.; Greigovieva, I.V.; Firslov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- López, V.; Sundaram, R.S.; Gómez-Navarro, C.; Olea, D.; Burghard, M.; Gómez-Herrero, J.; Zamora, F.; Kern, K. Chemical vapor deposition repair of graphene oxide: A route to highly-conductive graphene monolayers. Adv. Mater. 2009, 21, 4683–4686. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sunitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Mermoux, M.; Chabre, Y.; Rousseau, A. FTIR and 13C NMR study of graphite oxide. Carbon 1991, 29, 469–474. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Mermoux, M.; Chabre, Y. Formation of graphite oxide. Synth. Metals 1989, 34, 157–162. [Google Scholar] [CrossRef]
- Szabo, T.; Tombacz, E.; Illes, E.; Dekany, I. Enhanced acidity and pH-dependent surface charge characterization of successively oxidized graphite oxides. Carbon 2006, 44, 537–545. [Google Scholar] [CrossRef]
- Seredych, M.; Bendosz, T. Combine role of water and surface chemistry in relative adsorption of ammonia on graphite oxides. Langmuir 2010, 26, 5491–5498. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yamaguchi, D.; Suganuma, S.; Nakajima, K.; Kato, H.; Hayashi, S.; Hara, M. Adsorption-enhanced hydrolysis of β-1,4-glucan on graphene-based amorphous carbon bearing SO3H, COOH and OH groups. Langmuir 2009, 25, 5068–5075. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Huang, M.; Ma, H.L.; Zhang, Z.-Q.; Gao, J.M.; Zhu, Y.L.; Han, X.J.; Guo, X.Y. Preparation of a carbon based solid acid catalyst by sulfonationg activated carbon in a chemical reduction process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Zhang, L.; Wang, H.; Lv, P.; Yu, H. Sulfonated carbon nanotubes as a strong protonic acid catalyst. Carbon 2005, 43, 2405–2408. [Google Scholar] [CrossRef]
- Yu, H.; Jin, Y.; Li, Z.; Peng, F.; Wang, H. Synthesis and characterization of sulfonated single-walled carbon nanotubes and their performance as solid acid catalyst. J. Solid State Chem. 2008, 181, 432–438. [Google Scholar] [CrossRef]
- Liu, K.; Li, C.; Zhang, X.; Hua, W.; Yang, D.; Hu, J.; Yue, Y.; Gao, Z. Poly(styrene sulfonic acid)- grafted carbon nanotube as a stable protonic acid catalyst. Synth. Commun. 2010, 12, 217–221. [Google Scholar] [CrossRef]
- Salzman, C.G.; Liewellyn, S.A.; Tobias, G.; Ward, M.A.H.; Huh, Y.; Green, M.L.H. The role of carboxylated carbonaceous fragments in the functionalization and spectroscopy of a single walled carbon nanomaterial. Adv. Mater. 2007, 19, 883–887. [Google Scholar] [CrossRef]
- Wilson, K.; Clark, J.H. Solid acids and their use as environmentally friendly catalysts in organic synthesis. Pure Appl. Chem. 2000, 72, 1313–1319. [Google Scholar] [CrossRef]
- Clark, J.H. Solid acids for green chemistry. Acc. Chem. Res. 2002, 35, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; El-Hiti, G.A.; Jayne, A.J.; Butters, M. Acetylation of aromatic ethers using acetic anhydride over solid acid catalysts in a solvent-free system. Scope of the reaction for substituted ethers. Org. Biomol. Chem. 2003, 1, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Harmer, M.A.; Sun, Q. Solid acid catalysis using ion-exchange resins. Appl. Catal. A Gen. 2001, 221, 45–62. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by Heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, G.P.; Autino, J.C. Recent applications of heteropolyacids and related compounds in heterocycles synthesis. Mini-Rev. Org. Chem. 2009, 6, 359–366. [Google Scholar] [CrossRef]
- Palkovits, R.; Tajvidi, K.; Ruppert, A.M.; Procelewska, J. Heteropoly acids as efficient acid catalysts in the one-step conversion of cellulose to sugar alcohols. Chem. Commun. 2011, 47, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Kantam, L.M.; Ravindra, A.; Reddi, C.R.V.; Sreedar, B.; Choudary, B.M. Layered double hydroxide supported diisopropylamide, synthesis, characterization and application in organic reactions. Adv. Synth. Catal. 2006, 348, 569–578. [Google Scholar] [CrossRef]
- Tagusagawa, C.; Takagaki, A.; Takanabe, K.; Ebitani, K.; Hayashi, S.; Domen, K. Layered and nanosheet tantalum molybdate as strong solid acid catalysts. J. Catal. 2010, 270, 206–212. [Google Scholar] [CrossRef]
- Lomeda, J.R.; Doyle, C.D.; Kosynkin, D.V.; Hwang, W.-F.; Tour, J.M. Diazonium functionalization of surfactant-wrapped chemically converted graphene sheets. J. Am. Chem. Soc. 2008, 130, 16201–16206. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gong, K.; Xiao, P.; Xiao, M. Preparation and characterization of poly(vinyl acetate)-intercalated graphite oxide nanocomposite. J. Mater. Chem. 2000, 10, 933–935. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersion of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Zhang, B.; Ning, W.; Zhang, J.; Qiao, X.; Zhang, J.; He, J.; Liu, C.Y. Stable dispersions of reduced graphene oxide in ionic liquids. J. Mater. Chem. 2010, 20, 5401–5403. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Preparation of fully exfoliated graphite oxide nanoplatelets in organic solvents. J. Mater. Chem. 2007, 17, 3678–3680. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Jia, H.-P.; Bielawski, C.W. Graphene oxide: A convenient carbocatalyst for facilitating oxidation and hydration reactions. Angew. Chem. Int. Ed. 2010, 49, 6813–6816. [Google Scholar] [CrossRef]
- Pyun, J. Graphene oxide as catalyst: Application of carbon materials beyond nanotechnology. Angew. Chem. Int. Ed. 2011, 50, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Kumari, P.; Sinha, N.; Chauhan, P.; Chauhan, S.M.S. Isolation, synthesis and biomimetic reactions of metalloporphyrins in ionic liquids. Curr. Org. Synth. 2011, 8, 393–437. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T.; Chauhan, S.M.S. Meso-functional calix[4]pyrrole: A solution phase study of anion directed self-assembly. J. Inc. Phen. Macrocyc. Chem. 2011, 70, 249–255. [Google Scholar] [CrossRef]
- Lee, C.H. Versatilities of calix[4]pyrrole based anion receptors. Bull. Korean Chem. Soc. 2011, 32, 768–778. [Google Scholar] [CrossRef]
- Gale, P.A. From anion receptors to transporters. Acc. Chem. Res. 2011, 44, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Danil de Namor, A.F.; Khalife, R. Calix[4]pyrrole derivative: Recognition of fluoride and mercury ions and extracting properties of the receptor based new material. J. Phys. Chem. B 2008, 112, 15766–15774. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Tong, C.C.; Haynes, C.J.E.; Adeosun, O.; Gross, D.E.; Karnas, E.; Sedenberg, E.M.; Quesada, R.; Sessler, J.L. Octafluorocalix[4]pyrrole: A chloride/bicarbonate antiport agent. J. Am. Chem. Soc. 2010, 132, 3240–3241. [Google Scholar] [CrossRef] [PubMed]
- Stepanek, P.; Simak, O.; Novakova, Z.; Wimmer, Z.; Drasar, P. Asymmetrically substituted calix[4]pyrrole with chiral substituents. Org. Biomol. Chem. 2011, 9, 682–683. [Google Scholar] [CrossRef] [PubMed]
- Rohand, T.; Dolusic, E.; Ngo, T.H.; Maes, W.; Dahaen, W. Efficient synthesis of aryldipyrromethanes in water and their application in synthesis of corroles and dipyrromethanes. ARKIVOC 2007, x, 307–324. [Google Scholar]
- Shao, S.J.; Yu, X.D.; Cao, S.Q. Synthesis of calix[4]pyrroles: A class of new molecular receptor. Chinese Chem. Lett. 1999, 10, 193–194. [Google Scholar]
- Shao, S.; Wang, A.; Yang, M.; Jiang, S.; Xianda, Y. Synthesis of meso-aryl-substituted calix[4]pyrroles. Synth. Commun. 2001, 31, 1421–1426. [Google Scholar] [CrossRef]
- Gao, G.H.; Lu, L.; Gao, J.B.; Zhou, W.J.; Yang, J.G.; Yu, X.Y.; He, M.Y. One step synthesis of dipyrromethanes in presence of ionic liquid [Hmim]BF4. Chin. Chem. Lett. 2005, 16, 900–902. [Google Scholar]
- Kishan, M.R.; Srinivas, N.; Raghavan, K.V.; Kulkarni, S.J.; Sarma, J.A.R.P.; Vairamani, M. A novel, shape-selective, zeolite-catalyzed synthesis of calix(4)pyrroles. Chem. Commun. 2001, 2226–2227. [Google Scholar] [CrossRef]
- Kishan, M.R.; Rani, V.R.; Murty, M.R.V.S.; Sita Devi, P.; Kulkarni, S.J.; Raghavan, K.V. Synthesis of calixpyrroles and porphyrins over molecular sieve catalysts. J. Mol. Catal. A Chem. 2004, 223, 263–267. [Google Scholar] [CrossRef]
- Kishan, M.; Rani, V.R.; Kulkarni, S.J.; Raghavan, K.V. A new environmentally friendly method for the synthesis of calix(4)pyrroles over molecular sieve catalysts. J. Mol. Cat. A Chem. 2005, 237, 155–160. [Google Scholar] [CrossRef]
- Chauhan, S.M.S.; Garg, B.; Bisht, T. Syntheses of calix[4]pyrroles by amberlyst-15 catalyzed cyclocondensations of pyrrole with selected ketones. Molecules 2007, 12, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.M.S.; Bisht, T.; Garg, B. 1-Arylazo-5,5-dimethyl dipyrromethanes: Versatile chromogenic probes for anions. Sensor Actuat B Chem. 2009, 141, 116–123. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, G.; Liu, J.; Sun, X. Evaluation criteria for reduced graphene oxide. J. Phys. Chem. C 2011, 115, 11327–11335. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Yin, J. Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. ACS Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.I.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. Graphene oxide dispersion in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Majumdar, M.; Alemany, L.B.; Narayanan, T.N.; Ibarra, M.A. Engineered graphene oxide materials for application in water purification. ACS Appl. Mater. Interfaces 2011, 3, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Limited samples of compounds 3a and 4a are available from the authors. |

| Entry | Solvent | Catalyst | 2 | Conversion of pyrrole (%) | Yield of product (wt%) b,c | ||
|---|---|---|---|---|---|---|---|
| 3 | 4 | Other | |||||
| 1 | CH2Cl2 | GO | 2a | 99 | 99 | - | - |
| 2 | CH2Cl2 d | GO | 2a | 99 | 99 | - | - |
| 3 | CH2Cl2 | GO | 2b | 90 | 73 | 2 | 15 |
| 4 | CH2Cl2 d | GO | 2b | 86 | 70 | - | 9 |
| 5 | CH2Cl2 d | Zeolite HY[52] | 2b | 87.9 | 62.7 | - | 16.2 |
| 6 | CH2Cl2 d | HZSM-5(30)[52] | 2b | 69.6 | 53.0 | 10.7 | 5.9 |
| 7 | CH2Cl2 d | Al-MCM-41 | 2a | 95 | 12.3 | 70.3 | 12.4 |
| 8 | CHCl3 | AmberlystTM-15[55] | 2a | 99 | 83 | 14 e | |
| 9 | CHCl3 | GO | 2a | 99 | 97 | 2 | - |
| 10 | CCl4 | GO | 2a | 99 | 95 | 3 | - |
| 11 | Methanol | GO | 2a | 100 | 90 | 10 | - |
| 12 | CH3CN | GO | 2a | 98 | 80 | 13 | 5e |
| 13 | Ethylene glycol | GO | 2a | 98 | 34 | 7 | 57 |
| 14 | Acetone g | GO | 2a | 99 | 27 | 62 (50) f | 10e (6) f |
| Entry | Pyrrole:Acetone | Conversion of pyrrole (%) | Yield of product (wt%) b,c | ||
|---|---|---|---|---|---|
| 3 | 4 | Other | |||
| 1 | 1:1 | 93 | 92 | - | 1 |
| 2 | 2:1 | 97 | 97 | - | - |
| 3 | 5:1 | 99 | 98 | - | 1 |
| 4 | 1:1.5 | 98 | 97 | - | 1 |
| 5 | 1:5 | 100 | 96 | 3 | 1 |
| 6 | 1:10 | 100 | 84 | 8 | 2 d |
| Entry | Surfactant and salts | Time | Conversion of pyrrole (%) | Yield of product (wt%) b,c | ||
|---|---|---|---|---|---|---|
| 3 | 4 | Other | ||||
| 1 | SDS alone | 24 h | No reaction | - | - | - |
| 2 | SDS+GO | 1.5–3 h | 93 | 48 | 35 | 10 d |
| 3 | CTAB + GO | 24 h | 96 | 93 | - | 3 |
| 4 | PEG + GO | 24 h | 99 | 96 | - | 3 |
| 5 | Poly(sodium 4-styrenesulfonate) + GO | 24 h | 89 | 84 | 5 | - |
| 6 | Sodium methanesulfonate + GO | 24 h | 99 | 99 | - | - |
| 7 | Methanesulfonic acid (2 × 10−2 M) | 24 h | 99 | 79 | 2 | - |
| 8 | Sodium trifluoromethane sulfonate + GO | 24 h | 99 | 99 | - | - |
| Entry | Solvent | Time | Conversion of pyrrole (%) | Yield of product (wt%) b,c | ||
|---|---|---|---|---|---|---|
| (3) | (4) | Other | ||||
| 1 | Dichloromethane | 24 h | 99 | 98 | 1 | - |
| 2 | Methanol | 24 h | 99 | 94 | 5 | - |
| 3 | Acetone | 24 h | 100c | 52 | 25 | 10 d |
| 4 | Water | 24 h | 92 | 82 | - | 10 |
| 5 | Water + SDS | 24 h | 92 | 69 | 1 | 22 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Singh Chauhan, S.M.; Mishra, S. Use of Graphite Oxide and Graphene Oxide as Catalysts in the Synthesis of Dipyrromethane and Calix[4]pyrrole. Molecules 2011, 16, 7256-7266. https://doi.org/10.3390/molecules16097256
Singh Chauhan SM, Mishra S. Use of Graphite Oxide and Graphene Oxide as Catalysts in the Synthesis of Dipyrromethane and Calix[4]pyrrole. Molecules. 2011; 16(9):7256-7266. https://doi.org/10.3390/molecules16097256
Chicago/Turabian StyleSingh Chauhan, Shive Murat, and Sweta Mishra. 2011. "Use of Graphite Oxide and Graphene Oxide as Catalysts in the Synthesis of Dipyrromethane and Calix[4]pyrrole" Molecules 16, no. 9: 7256-7266. https://doi.org/10.3390/molecules16097256
APA StyleSingh Chauhan, S. M., & Mishra, S. (2011). Use of Graphite Oxide and Graphene Oxide as Catalysts in the Synthesis of Dipyrromethane and Calix[4]pyrrole. Molecules, 16(9), 7256-7266. https://doi.org/10.3390/molecules16097256